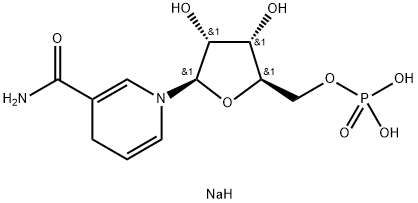
β-nicotinamide mononucleotide, reduced form, disodium salt(NMNH): Theoretical Study, Bioactivity and Application
General description
The interaction of reduced nicotinamide mononucleotide (NMNH), constituting one half of NADH, with the wild-type and alpha D195E proton-pumping nicotinamide nucleotide transhydrogenase from Escherichia coli was investigated. Reduction of thio-NADP(+) by NMNH was catalysed at approximately 30% of the rate with NADH. Other activities including proton pumping and the cyclic reduction of 3'-acetyl-pyridine-NAD(+) by NMNH in the presence of NADP+ were more strongly inhibited. The alpha D195 residue is assumed to interact with the 2'-OH moiety of the adenosine-5'-phosphate, i.e., the second nucleotide of NADH. Mutation of this residue to alpha D195E resulted in a 90% decrease in activity with NMNH as well as NADH as substrate, suggesting that it produced global structural changes of the NAD(H) binding site. The results suggest that the NMN moiety of NADH is a substrate of transhydrogenase, and that the adenine nucleotide is not required for catalysis or proton pumping.
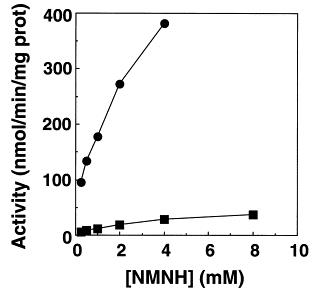
Fig. 1. Reduction of t-NADP+ by NMNH catalyzed by wild-type and αD195E mutant transhydrogenases. The assays were carried out as described in Section 2. (?) wild-type; (■) αD195E.
Experimental and Theoretical Study
Ab Initio Studies of NMNH(2-) Conformers in Water-Methanol Solutions
Ab initio studies of the structure of reduced beta-nicotinamide d-ribonucleotide (NMNH(2-)) conformations in water and methanol solutions have been carried out for clarifying the role of the phosphate groups in fluorescence parameters of the NMNH(2-) molecule and the reduced beta-nicotinamide adenine dinucleotide (NADH) molecule. Relaxed potential energy surfaces as a function of the dihedral rotation angle of the amide group in the NMNH(2-) molecule were calculated in the ground electronic state and the first excited electronic state to better understand the effect of phosphate groups on the nonradiative decay rates in the nicotinamide chromophore groups. The differences in the weighting coefficients in the biexponential fluorescence signals for NMNH(2-) and NADH molecules in solution were explained. A strong hydrogen bonding between the amide hydrogen atom and the nearest oxygen O- atom from the phosphate group was detected by ab initio calculations for the folded NMNH(2-) conformations in the ground electronic state at trans configurations of the nicotinamide ring. This hydrogen bonding turned out to be much weaker for the first excited electronic state. These calculated data show that, after optical excitation of the NMNH(2-) molecule, a rapid change in the geometry of the molecule is possible. The strong interaction of the phosphate group with the amide group in NMNH(2-) molecules in aqueous solution leads to the predominance of the folded NMNH(2-) conformations and trans configurations of the nicotinamide ring. This explains the reason for the dominance of one fluorescence decay time of NMNH(2-) in the aqueous solution. Based on these data, an important conclusion can be drawn that the contribution of the exponent with the short decay time tau 0.28 ns to the fluorescence signal of NMNH(2-), NADH, and NADPH molecules is related to the trans configuration of the nicotinamide ring [1].
Bioactivity
Potently Enhances NAD(+) and Suppresses Glycolysis, the TCA Cycle, and Cell Growth
Decreased cellular NAD(+) levels are causally linked to aging and aging-associated diseases. NAD(+) precursors in oxidized form such as nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) have gained much attention and been well studied for their ability to restore NAD(+) levels in model organisms. Less is known about whether NAD(+) precursors in reduced form can also efficiently increase the tissue and cellular NAD(+) levels and have different effects on cellular processes than NMN or NR. In the present study, we developed a chemical method to produce dihydronicotinamide mononucleotide (NMNH), which is the reduced form of NMN. We demonstrated that NMNH was a better NAD(+) enhancer than NMN both in vitro and in vivo, mediated by nicotinamide mononucleotide adenylyltransferase (NMNAT). Additionally, NMNH increased the reduced NAD (NADH) levels in cells and in mouse livers. Metabolomic analysis revealed that NMNH inhibited glycolysis and the TCA cycle. In vitro experiments demonstrated that NMNH induced cell cycle arrest and suppressed cell growth. Nevertheless, NMNH treatment did not cause an observable difference in mouse weight. Taken together, our work demonstrates that NMNH is a potent NAD(+) enhancer and suppresses glycolysis, the TCA cycle, and cell growth [2].
Nicotinamide adenine dinucleotide (NAD(+)) homeostasis is constantly compromised due to degradation by NAD(+)-dependent enzymes. NAD(+) replenishment by supplementation with the NAD(+) precursors nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) can alleviate this imbalance. However, NMN and NR are limited by their mild effect on the cellular NAD(+) pool and the need of high doses. Here, we report a synthesis method of a reduced form of NMN (NMNH), and identify this molecule as a new NAD(+) precursor for the first time. We show that NMNH increases NAD(+) levels to a much higher extent and faster than NMN or NR, and that it is metabolized through a different, NRK and NAMPT-independent, pathway. We also demonstrate that NMNH reduces damage and accelerates repair in renal tubular epithelial cells upon hypoxia/reoxygenation injury. Finally, we find that NMNH administration in mice causes a rapid and sustained NAD(+) surge in whole blood, which is accompanied by increased NAD(+) levels in liver, kidney, muscle, brain, brown adipose tissue, and heart, but not in white adipose tissue. Together, our data highlight NMNH as a new NAD(+) precursor with therapeutic potential for acute kidney injury, confirm the existence of a novel pathway for the recycling of reduced NAD(+) precursors and establish NMNH as a member of the new family of reduced NAD(+) precursors [3].
Application
On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids
Degradation of the reduced pyridine nucleotides NMNH and NADH by HOCl involves two distinct stages: a fast reaction, k = 4.2 x 10(5) M-1 s(-1), leads to generation of stable pyridine products (Py/Cl) with a strong absorption band at 275 nm (epsilon = 12.4 x 10(3) M(-)1 cm(-1) in the case of NMNH); secondarily, a subsequent reaction of HOCl, k = 3.9 x 10(3) M-1 s(-1), leads to a complete loss of the aromatic absorption band of the pyridine ring. HOBr and HOI(I-2) react similarly. Apparent rate constants of the primary reactions of HOX species with NMNH at pH 7.2 increase in the order HOCl (3 x 10(5) M-1 s(-1)) < HOBr(similar to 4 x 10(6) M-1 s(-1)) < HOI(I-2)(similar to 6.5 x 10(7) M-1 s(-1)). HOBr reacts fast also with the primary product Py/Br, k similar to 9 x 10(5) M-1 s(-1), while the reactions of HOI and I-2 with Py/I are slower, similar to 1.4 x 10(3) M-1 s(-1) and >6 x 10(3) M-1 s(-1), respectively. Halogenation of the amide group of NMN+ by HOX species is many orders of magnitude slower than oxidation of NMNH. Taurine inhibits HOCl-induced oxidation of NADH, but HOBr-induced oxidation is not inhibited because the taurine monobromamine rapidly oxidizes NADH, and oxidation by HOI(I-2) is not inhibited because taurine is inert toward HOI(I-2), Also sulfu
You may like
See also

US $1.00/KG2025-10-14
- CAS:
- 108347-85-9
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 20T

US $0.00/kg2025-08-19
- CAS:
- 108347-85-9
- Min. Order:
- 1kg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 2tons