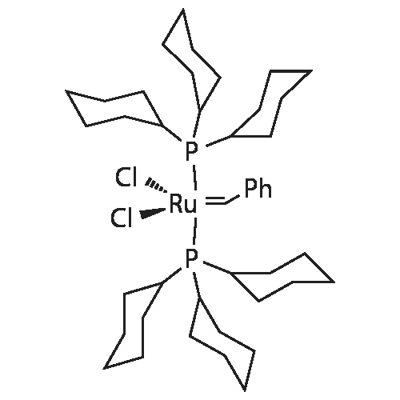
Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium: Versatile Catalyst in Olefin Metathesis
Robert H Grubbs is celebrated for his path breaking discovery of a series of ruthenium carbene based organometallic catalysts, Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium, well known as Grubbs catalysts. Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium is widely used by chemists across the world for carrying out a variety of olefin metathesis reactions under mild and ambient conditions.

Synthesis of Macrocyclic Tetralactones using Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
Ring-closing metathesis (RCM) reactions have become one of the most versatile and efficient methods for constructing many carbo- and heterocyclic compounds. They have been extensively used as a key step in a number of natural product syntheses to install a cyclic structure. Recently, we have reported the synthesis of macrocyclic tetralactones via DCC/DMAP cyclization of dicarboxylic acids. Since there has been no other literature report toward the synthesis of macrocyclic tetralactones, we herein report a successful method for macrocyclic tetralactones with different ring sizes via RCM reaction. We next performed the RCM reactions of the compounds 2 having a symmetrical diolefinic end using Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium. Thus, compound 2a was stirred with 5 mol % of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium and 2 equiv of CsCl under an inert atmosphere to afford 21-membered macrocyclic tetralactone in 64% yield.[1]
To exploit this interesting result, the diallylated compound 2b was also subjected to RCM reaction using 5 mol % of the Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium to afford the tetralactone as a single isomer based on the proton NMR having 20 atoms in the cyclic core. In conclusion, this work described the preparation of symmetrical bis-olefinic compounds by tethering several spacers and their RCM reactions providing an expedient strategy for the synthesis of symmetrical macrocyclic tetralactones. This methodology brings another application of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium for the synthesis of macrocyclic tetralactones having different ring sizes and spacers. These macrocyclic compounds are expected to be potentially important to the biologically significant materials and supramolecular chemistry.
Si–C bond activation in the reaction of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
The first generation Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium [Cl2{P(C6H11)3}2RuC(H)Ph] reacts efficiently with alkynylsilanes in the presence of water to give the styryl carbene complex [Cl2{P(C6H11)}2 RuC(CHCHPh)H] and disiloxane. The activity of the family of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium in metathesis transformations of carbon–carbon triple bonds such as in enyne metathesis,1alkyne2 and diynepolymerization is well known. Until now, only a few reports have appeared on the reactivity of alkynes in equimolar reactions with Grubbs type ruthenium carbene complexes. Grubbs reported the reaction of electron-rich disubstituted alkynes with [Cl2{P(C6H11)3}(H2IMes)RuC(H)Ph] giving phosphane-free η3-vinylcarbene complexes. When we treated a first generation Grubbs catalyst (1) with stoichiometric amounts of ethynyltrimethylsilane in benzene at 40 °C in the presence of water, a gradual change in the colour of the solution from violet to red was observed. GC-MS analysis of the reaction mixture indicated the conversion of silylacetylene and the formation of hexamethyldisiloxane.[2]
The reactivity of alkynes bearing a silyl group directly attached to the carbon–carbon triple bond towards Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium has not been studied in detail. Only a few examples of the activity of Grubbs catalysts in enynering-closing metathesis (RCM) and in cross metathesis (CM) of alkynylsilanes with olefins are known. In this communication we report on the cleavage of the carbon–silicon bond accompanying the reaction of a first generation Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium with ethynylsilanes in the presence of water. The known examples of the cleavage of the Si–C bond in silylacetylenes by transition metal complexes include 1,2-silyl migration accompanying the formation of vinylidene complexes of various transition metals, palladium catalysed cross-coupling silylacetylenes with organic halides,σ-bond metathesis of silylalkynes by uranium metallocene as well as some other platinum, rhodium or nickel catalysed reactions.
Statistical Ring Opening Metathesis Copolymerization of Norbornene
Ring Opening Metathesis Polymerization (ROMP), a relatively new tool in the field of polymer chemistry, has emerged as a powerful and broadly-applicable method for synthesizing macromolecular materials. Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium are more accessible, compatible with a wide range of solvents, and tolerant to ubiquitous impurities such as oxygen and water. For these reasons, they have found extensive use in organic and polymer chemistry and afford polymers with novel mechanical, electronic and, more recently, biological properties. Statistical copolymers of NBE and CP were synthesized in a controlled manner via ROMP using Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium in the presence and absence of triphenylphosphine. In the presence of phosphine the copolymerizations were conducted at room temperature and at 0 °C in the absence of phosphine.[3]
References
[1]Muthusamy, Sengodagounder et al. “New approach to the synthesis of macrocyclic tetralactones via ring-closing metathesis using Grubbs' first-generation catalyst.” The Journal of organic chemistry vol. 72,4 (2007): 1495-8. doi:10.1021/jo062043p
[2]Powa?a, Beata et al. “Si-C bond activation in the reaction of first generation Grubbs' catalyst with alkynylsilanes - formation of [Cl(2){P(C(6)H(11))(3)}(2)Ru(=CHCH=CHPh)] anddisiloxanes.” Dalton transactions (Cambridge, England : 2003) vol. 39,8 (2010): 1923-5. doi:10.1039/b924945c
[3]Nikovia C, Maroudas AP, Goulis P, Tzimis D, Paraskevopoulou P, Pitsikalis M. Statistical Ring Opening Metathesis Copolymerization of Norbornene and Cyclopentene by Grubbs' 1st-Generation Catalyst. Molecules. 2015 Aug 27;20(9):15597-615. doi: 10.3390/molecules200915597. PMID: 26343620; PMCID: PMC6331872.
You may like
See also
Lastest Price from Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium manufacturers

US $0.00-0.00/G2025-03-03
- CAS:
- 172222-30-9
- Min. Order:
- 1G
- Purity:
- 98%
- Supply Ability:
- 250G
US $116.00-407.00/g2025-02-08
- CAS:
- 172222-30-9
- Min. Order:
- 5g
- Purity:
- 0.98
- Supply Ability:
- 25kg