(+)-Camptothecin: A Key Player in Chemotherapy
Introduction
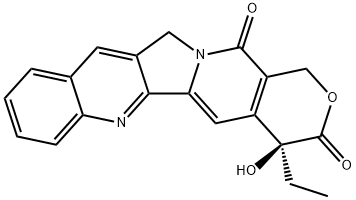
(+)-Camptothecin, a potent alkaloid with significant pharmaceutical importance, has garnered attention in chemotherapy due to its remarkable anti-cancer properties. Derived from the bark and stem of the Chinese tree Camptotheca acuminata, this compound has fascinated chemists and pharmacologists alike since its discovery in the 1960s. Its intricate molecular structure, characterized by a pentacyclic core and a lactone ring, underscores its potent biological activity against various types of cancers. Researchers have explored its mechanism of action, focusing on its ability to inhibit topoisomerase I, a pivotal enzyme involved in DNA replication and transcription processes within cancer cells. This specific mode of action has positioned (+)-Camptothecin as a promising therapeutic agent against colorectal, ovarian, and small-cell lung cancers, among others. As the pharmaceutical community continues to unravel its complexities, (+)-Camptothecin remains a beacon of hope in the quest for effective cancer treatments, driving forward innovations in medicinal chemistry and oncology research.

Figure 1 Characteristics of (+)-camptothecin
Nature of (+)-Camptothecin
(+)-Camptothecin, often abbreviated as CPT, is a naturally occurring quinoline alkaloid characterized by its potent pharmacological properties. Its pentacyclic structure, which includes a fused pyrrole ring system, contributes significantly to its biological activity by facilitating interactions with topoisomerase I. Initially isolated from the Chinese tree Camptotheca acuminata in the 1960s, (+)-Camptothecin has spurred extensive research into its chemical synthesis, structural modifications, and therapeutic applications in oncology. This compound continues to be a focal point of exploration, driving advancements in cancer treatment strategies worldwide.
Main Components
Chemically, (+)-Camptothecin consists of a lactone ring fused to a pentacyclic core structure comprised of six fused rings. This complex architecture is essential to its pharmacological activity, with the (+)-enantiomer exhibiting optimal efficacy. The molecule's specific spatial arrangement allows it to interact selectively with topoisomerase I, an enzyme critical for maintaining DNA structure during replication and transcription. This interaction results in the formation of a stable ternary complex between (+)-Camptothecin, DNA, and topoisomerase I, leading to DNA strand breaks and subsequent cytotoxic effects in cancer cells. The precise stereochemistry and structural features of (+)-Camptothecin continue to be studied to further elucidate and enhance its therapeutic potential in oncology.
Applications in Chemotherapy
The primary application of (+)-Camptothecin lies in oncology, where it functions as a potent anti-cancer agent by selectively inhibiting topoisomerase I. This enzymatic inhibition disrupts DNA replication and transcription processes in cancer cells, inducing breaks in DNA strands and ultimately triggering apoptosis in rapidly dividing cells. Its effectiveness spans various cancer types, including colorectal, ovarian, and small-cell lung cancers, where (+)-Camptothecin's ability to target proliferating cells offers a promising therapeutic strategy. Ongoing research aims to optimize delivery methods and combination therapies to enhance efficacy and minimize side effects, underscoring its pivotal role in modern chemotherapy regimens.
Storage Considerations
Proper storage of (+)-Camptothecin is essential to maintain its stability and efficacy. The compound is sensitive to light and moisture, which can degrade its potency over time. Therefore, it is typically stored in tightly sealed amber glass containers at temperatures below -20°C. This ensures minimal exposure to light and moisture, preserving its chemical integrity for extended periods.
Conclusion
In conclusion, (+)-Camptothecin stands as a cornerstone in the realm of chemotherapy, offering a targeted approach to combating various forms of cancer. Its intricate molecular structure, potent biological activity, and specific mode of action underscore its significance in pharmaceutical research and clinical applications. As research continues to unfold, further insights into the chemistry and pharmacology of (+)-Camptothecin promise to expand its therapeutic potential, offering hope in the ongoing battle against cancer.
This article has provided a comprehensive overview of (+)-Camptothecin, highlighting its chemical composition, therapeutic applications, and optimal storage practices. For professionals in the chemistry field, understanding these aspects is pivotal in harnessing the full potential of (+)-Camptothecin in medicinal chemistry and oncology.
[1] Thomas C J, Rahier N J, Hecht S M. Camptothecin: current perspectives[J]. Bioorganic & medicinal chemistry, 2004, 12(7): 1585-1604.
[2] Li Q Y, Zu Y G, Shi R Z, et al. Review camptothecin: current perspectives[J]. Current medicinal chemistry, 2006, 13(17): 2021-2039.
References:
[1] CRAIG J. THOMAS S M H Nicolas J Rahier. Camptothecin: current perspectives[J]. Bioorganic & Medicinal Chemistry, 2004, 12 7: 1571-1810. DOI:10.1016/j.bmc.2003.11.036.[2] QING-YONG LI. Review camptothecin: current perspectives.[J]. Current medicinal chemistry, 2006, 13 17. DOI:10.2174/092986706777585004.
You may like
Lastest Price from (+)-Camptothecin manufacturers

US $10.00/ASSAYS2025-08-22
- CAS:
- 7689-03-4
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/kg2025-04-21
- CAS:
- 7689-03-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg