网站主页
化工产品目录
生物化工
生化试剂
激动剂抑制剂
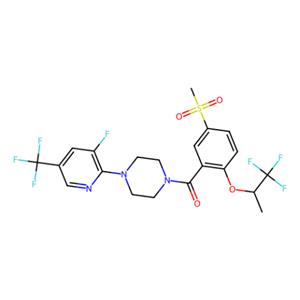
(S)-[4-(3-氟-5-三氟甲基吡啶-2-基)哌嗪-1-基][5-(甲磺酰基)-2-(2,2,2-三氟-1-甲基乙氧基)苯基]甲酮
比托派汀
比托派汀|T6788|TargetMol
Bitopertin
845614-11-1
845614-11-1
¥248
1mg
起订
¥355
2mg
起订
¥579
5mg
起订
上海 更新日期:2025-11-17
产品详情:
- 中文名称:
- 比托派汀
- 英文名称:
- Bitopertin
- CAS号:
- 845614-11-1
- 品牌:
- TargetMol
- 产地:
- 美国
- 保存条件:
- store at low temperature | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature.
- 纯度规格:
- 98.57%
- 产品类别:
- 抑制剂
- 货号:
- T6788
公司简介
TargetMol Chemicals Inc. 总部位于马萨诸塞州波士顿,致力于为全球生化领域科学家的研究提供专业的产品和服务。TargetMol?品牌的客户群分布于40多个国家和地区,已发展成为全球知名的化合物库和小分子化合物研究供应商。 TargetMol?可提供160多种满足不同需求的化合物库,以及多种类型的生化试剂产品,包括12000多种抑制剂、16000多种天然产物和各类多肽、抗体、生命科学试剂盒等,此外,我们还建设有CADD(计算机辅助药物设计)研究中心、药理实验室、药化合成平台三大技术中心,全方位满足客户的定制需求。 凭借我们优质的产品和服务、快速高效的全球供应链和专业的技术支持,我们将有效帮助您缩短研发周期,取得更成功的结果。
| 成立日期 | (13年) |
| 注册资本 | 566.265100万人民币 |
| 员工人数 | 100-500人 |
| 年营业额 | ¥ 1亿以上 |
| 经营模式 | 贸易,工厂,试剂,定制,服务 |
| 主营行业 | 天然产物,生化试剂,分子生物学,分子砌块,生物技术服务 |
比托派汀相关厂家报价
-

- 托比司他
- 台州开创生物医药有限公司 VIP
- 2026-03-05
- 询价
-

- 比拉斯汀
- 江西瑞威尔生物科技有限公司 VIP
- 2026-03-05
- 询价
-

- 比拉斯汀
- 沧州恩科医药科技有限公司 VIP
- 2026-03-07
- 询价
-
- aladdin 阿拉丁 B127005 Bitopertin,非竞争性抑制剂 845614-11-1 ≥98%
- 上海阿拉丁生化科技股份有限公司 VIP
- 2025-11-14
- ¥858.90
-

- 845614-11-1 Bitopertin原料药
- 武汉贝尔卡生物医药有限公司
- 2024-04-08
- 询价


