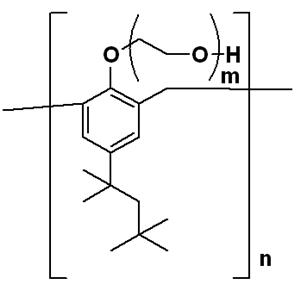
| Name | Tyloxapol |
| Description | Tyloxapol (Triton WR1339) is a non-ionic liquid polymer utilized as a surfactant. |
| Cell Research | HEK293 cells growing at 40% confluency are exposed to different test dispersions of SLP or their individual components for 48 h and observed the alterations in cellular morphology.(Only for Reference) |
| In vitro | Tyloxapol is generally regarded as a safe stabilizer. In some studies, it is reported to causes cytotoxicity in epithelial and red blood cells, induces lysis of human Jurkat T-lymphoblasts and the apoptosis in RAW 264.7 murine macrophage-like cells and NIH/3T3 mouse fibroblast cells. These indications of cytotoxicity, however, do not reflect the in vivo use of Tyloxapol, since it is rarely used alone in clinical applications[3]. |
| In vivo | A single intravenous injection of tyloxapol at dose of 400 mg/kg body weight shows three distinctive phases, sharp linear increment, slow linear increment and slow decrement of plasma lipids toward the basal levels[1]. The treatment of tyloxapol enhannces the pulmonary absorption of rh-insulin and increases the absorption of inhaled insulin in diabetic rats. It might significantly increase the hypoglycemic effect of intratracheally administered insulin in diabetic rats but does not change the LDH activity[2]. |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : 25 mg/mL, Sonication is recommended.
DMSO : 38 mg/mL
|
| Keywords | inhibit | Triton WR-1339 | Tyloxapol | Triton WR 1339 | Inhibitor |
| Inhibitors Related | Sodium 4-aminosalicylate dihydrate | Sodium Houttuyfonate | Lidocaine | Diethylmaleate | Glucosamine | sodium lauroyl-α-hydroxyethyl sulfonate | Lidocaine hydrochloride | 5-Aminosalicylic Acid | Indole-3-carbinol | Diallyl disulfide | Caffeic Acid Phenethyl Ester | Sodium salicylate |
| Related Compound Libraries | Anti-Obesity Compound Library |

 United States
United States