Overview.
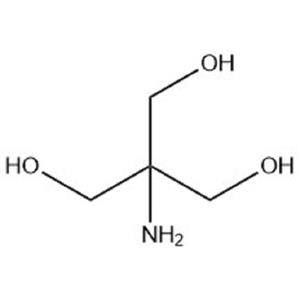
The Chinese name of Tris is Trimethylaminomethane; Aminobutriol; Bradykinin; 2-Amino-2-(hydroxymethyl)-1,3-propanediol. It is a white crystal or powder. Soluble in ethanol and water, slightly soluble in ethyl acetate, benzene, insoluble in ether, carbon tetrachloride, corrosive to copper, aluminum, irritating chemicals.
Indications.
Aminobutriol is a sodium-free amino buffer base, reacts with H2CO3 in body fluids to reduce H2CO3 and at the same time generates HCO32-, which can take up hydrogen ions and correct acidemia, its effect is strong and can penetrate cell membranes, commonly used in acute metabolic and respiratory acidemia.
Buffering properties.
Tris is a weak base with a pKa of 8.1 at 25°C. According to buffering theory, the effective buffering range of Tris buffer is between pH 7.0 and 9.2. The pH of aqueous solution of Tris base is around 10.5, and the pH buffer can be obtained by adding hydrochloric acid to adjust the pH to the desired value. However, attention should also be paid to the effect of temperature on the pKa of Tris.
Applications.
Tris is widely used in acute metabolic and respiratory acidemia and is an alkaline buffer with good buffering effect on metabolic acidosis and enzymatic activity reactions.
Tris is commonly used as a biological buffer, often formulated to pH 6.8, 7.4, 8.0, 8.8. Its structural formula, pH value varies greatly with temperature. In general, the pH value decreases by 0.03 for each degree of temperature increase.
Tris is widely used in the preparation of buffers for biochemistry and molecular biology experiments. For example, Tris is used in TAE and TBE buffers (for nucleic acid solubilization), which are commonly used in biochemistry experiments, and can be condensed with aldehydes because of its amino group.
Tris is a weak base with a pKa of 8.1 at room temperature (25°C); according to buffering theory, the effective buffering range of Tris buffers is between pH 7.0 and 9.2.
The pH of aqueous solution of Tris base is around 10.5. Generally, the buffer of this pChemicalbookH value can be obtained by adding hydrochloric acid to adjust the pH to the desired value. However, the effect of temperature on the pKa of Tris should also be noted.
Since Tris buffer is a weakly basic solution, DNA will be deprotonated in such a solution, thus increasing its solubility. A "TE buffer" is often made by adding EDTA to the Tris hydrochloric acid buffer, which is used for DNA stabilization and storage. If the pH-adjusting acid solution is replaced with acetic acid, a "TAE buffer" (Tris/Acetate/EDTA) is obtained, while replacing it with boric acid gives a "TBE buffer" (Tris/Borate/EDTA). . These two buffers are commonly used in nucleic acid electrophoresis experiments.
TAE, TBE, etc. prepared from Tris are the most commonly used reagents for DNA electrophoresis, while TE (pH 8.0) is mainly used to solubilize DNA (TE is Tris plus EDTA together.) 1MTris-HCl6.8 and 1.5MTris-HCl8.8 are the most commonly used reagents for SDS-PAGE.
Translated with www.DeepL.com/Translator (free version)

 China
China