| Chemical Properties | translucent crystals or white powder |
| Chemical Properties | Sodium thiosulfate occurs as odorless and colorless crystals, a crystalline powder or granules. It is efflorescent in dry air and deliquescent in moist air. |
| Uses | Titrimetry. |
| Uses | Sodium thiosulfate pentahydrate is used as an analytical reagent in laboratories. It is used in photographic processing and in gold extraction. Its alkylated derivatives, S-alkylthiosulfonates, are known as Bunte salts. It is involved in the preparation of thioglycolic acid. Furthermore, it is used to dechlorinate tap water, for removing free bromine from solutions and to check the pH of bleach solutions with liquid indicators. Other uses include tanning of leather; as an active component in hand warmers and chemical heating pads and as an anionic surfactant to aid in the dispersion of pharmaceutical preparations. |
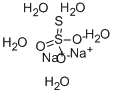
| Definition | ChEBI: Sodium thiosulfate pentahydrate is a hydrate consisting of sodium thiosulfate with 5 mol eq. of water. It has a role as an antidote to cyanide poisoning, a nephroprotective agent and an antifungal drug. It contains a sodium thiosulfate. |
| Production Methods | On an industrial scale, sodium thiosulfate is produced chiefly from liquid waste products of sodium sulfide or sulfur dye manufacture. Small-scale synthesis is done by boiling an aqueous solution of sodium sulfite with sulfur. |
| Brand name | Sulfactol (Sterling Winthrop). |
| Pharmaceutical Applications | Sodium thiosulfate is used as an antioxidant in pharmaceuticals (ophthalmic, intravenous, and oral preparations). It has also been used for its antifungal properties and as a reagent in analytical chemistry. |
| Safety | Sodium thiosulfate is used in ophthalmic, intravenous, and oral pharmaceutical preparations. Apart from osmotic disturbances, sodium thiosulfate is relatively nontoxic. It is moderately toxic by the subcutaneous route and mildly irritating to respiratory tract and skin. Large oral doses have a cathartic action.
LD50 (IP, mouse) 5.6 g/kg
LD50 (IV, mouse) 2.4 g/kg |
| storage | Sodium thiosulfate decomposes on heating. The bulk powder should be stored in a cool place, and the container should be kept tightly closed in a dry and well-ventilated place. It should not be stored near acids. |
| Purification Methods | Crystallise it from EtOH/H2O solutions or from water (0.3mL/g) below 60o by cooling to 0o, and dry it at 35o over P2O5 under vacuum. [Foerster & Mommsen Chem Ber 57 258 1924.] This salt is used as a secondary standard in volumetric analysis [Kilpatrick J Am Chem Soc 45 2132 1923], and is used as “Hypo” in photography [Hargreaves & Dunningham J Soc Chem Ind 42 147T 1923.] |
| Incompatibilities | Sodium thiosulfate is incompatible with iodine, with acids, and with lead, mercury, and silver salts. It may reduce the activity of some preservatives, including bronopol, phenylmercuric salts, and thimerosal. |
| Regulatory Status | GRAS listed. Included in the FDA Inactive Ingredients Database (IV solutions; ophthalmic solutions and suspensions; oral capsules, solutions, and tablets). Included in the Canadian List of Acceptable Non-medicinal Ingredients. |

 Japan
Japan






 Company information
Company information Advantage
Advantage


