Rimegepant Impurity
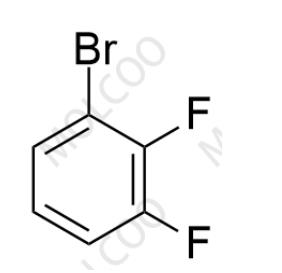
Product Code:R046076
English Name:Rimegepant Impurity 76
English Alias:1-bromo-2,3-difluorobenzene
CAS No.:38573-88-5
Molecular Formula:C₆H₃BrF₂
Molecular Weight:192.99
High-Purity Guarantee:Confirmed by HPLC (≥99.0%), combined with multiple techniques such as NMR (1H, 13C), HRMS, and elemental analysis, providing an accurate standard for Rimegepant impurity analysis.
Good Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.2% in common organic solvents (such as acetonitrile, toluene) within 6 months, ensuring stable and reliable experimental data.
Quality Control Testing:Used for UPLC-MS/MS or GC-MS detection of Impurity 76 in Rimegepant API and formulations, strictly controlling impurity content to meet ICH Q3A and Q3B standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitor the residue or formation of 1-bromo-2,3-difluorobenzene during Rimegepant synthesis. Reduce impurity residue by over 60% by adjusting the halogenation reaction temperature (e.g., 0 - 5℃), reaction time, and raw material ratio.
Method Validation:Serves as a standard for developing impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.005 ng/mL) to meet the strict requirements of regulatory authorities for detection methods.
Rimegepant, a calcitonin gene-related peptide (CGRP) receptor antagonist, is used for treating migraines. Impurity 76 (1-bromo-2,3-difluorobenzene) may remain as a raw material or be generated during synthesis steps such as halogenation and substitution. Its bromine and fluorine atoms may affect the drug's stability, safety, and subsequent metabolism. With the increasing strict requirements of global regulatory agencies (such as FDA and EMA) for drug impurities, the study of this impurity has become a crucial part of ensuring the quality of Rimegepant drugs and patient safety.
Detection Technology:High-sensitivity GC-MS/MS technology, equipped with a DB-5MS column (30m×0.25mm×0.25μm) and selected ion monitoring (SIM) mode, achieves separation within 5 minutes, with an LOD as low as 0.002 ng/mL, enabling precise detection of trace impurities.
Formation Mechanism:Studies indicate that this impurity is mainly formed during the benzene ring halogenation reaction. Excessive reaction temperature or an excess of brominating and fluorinating agents will increase its content. It can be effectively inhibited by optimizing reaction conditions, such as using a low-temperature dropwise addition method and precisely controlling reagent dosages.
Safety Evaluation:In vitro cytotoxicity tests show that the IC₅₀ of this impurity against HEK293 cells is 210.5 μM (Rimegepant IC₅₀ = 15.2 μM). Although less toxic than the main drug, its content in drugs still requires strict control. Currently, long-term stability tests are being carried out to systematically monitor its stability under different humidity, light, and temperature conditions.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China