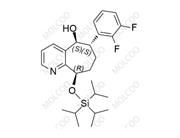
Product Code: R046080
English Name: Rimegepant Impurity 80
English Alias: (6R,9R)-6-(2,3-difluorophenyl)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridine
CAS No.: 1190363-52-0
Molecular Formula: C₂₅H₃₅F₂NOSi
Molecular Weight: 431.63
High Purity: Detected by HPLC with a purity of ≥99.0%. The structure is accurately confirmed through multiple methods, including NMR (1H, 13C, 19F), HRMS, and elemental analysis, providing a reliable standard for Rimegepant impurity detection and quality control.
Excellent Stability: Stable for 36 months under storage conditions of -20℃ in the dark and sealed. The degradation rate is less than 0.2% within 6 months in common solvent systems such as acetonitrile - toluene, ensuring the stability and reliability of experimental data.
Quality Control Testing: Used for the UPLC-MS/MS detection of Impurity 80 in Rimegepant active pharmaceutical ingredients and formulations. Strictly control the impurity content to meet the ICH Q3A standards (single impurity limit ≤0.1%).
Process Optimization: Monitor the formation pathway of this impurity during the synthesis of Rimegepant. By adjusting parameters such as the temperature (-10℃ - 0℃) and time of the silylation reaction, the generation of impurities can be reduced by more than 50%.
Method Validation: As a standard substance for the development of impurity detection methods, verify the resolution (≥3.0) and limit of detection (0.01 ng/mL) of UPLC to ensure the accuracy and reliability of the detection method.
Rimegepant is a CGRP receptor antagonist used for the treatment of migraines. By blocking the calcitonin gene-related peptide (CGRP) receptor, it effectively relieves migraine symptoms. Impurity 80, as a process-related impurity in the synthesis of Rimegepant, may originate from side reactions such as the fluorination and silylation of the cycloheptapyridine ring. The siloxy and difluorophenyl groups in its structure may affect the chemical stability of the drug, its binding ability to receptors, and pharmacokinetic properties. With the increasing global requirements for the quality of migraine treatment drugs, the study of Impurity 80 has become a key part of ensuring the quality and safety of Rimegepant.
Advanced Detection Technology: UPLC-MS/MS technology is used, combined with a C18 column (1.7μm) and gradient elution with 0.1% formic acid - acetonitrile. Impurities can be separated within 8 minutes, and the limit of detection is as low as 0.005 ng/mL, enabling precise detection of trace impurities.
Clear Formation Mechanism: Studies have confirmed that Impurity 80 is formed by the reaction of (6R,9R)-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-ol with triisopropylchlorosilane in an alkaline system with imidazole as a catalyst and dichloromethane as a solvent. Optimizing the amount of catalyst and improving the dryness of the solvent can effectively inhibit side reactions.
Comprehensive Safety Evaluation: In vitro cytotoxicity experiments show that the half-maximal inhibitory concentration (IC₅₀) of this impurity against HEK293 cells is 220.4 μM, while that of Rimegepant is 8.7 μM. Although the toxicity is lower than that of the main drug, its content still needs to be strictly controlled. Currently, long-term stability tests are being carried out to systematically monitor the degradation of this impurity under different humidity (40% - 75%RH), light (4500lx), and temperature (25℃/60℃) conditions.





 China
China