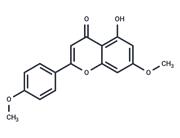
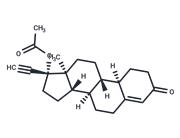
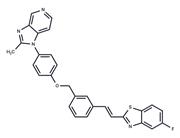
| Name | Norethindrone |
| Description | Norethindrone (Norethisterone) is a synthetic progestational hormone with actions similar to those of PROGESTERONE but functioning as a more potent inhibitor of ovulation. It has weak estrogenic and androgenic properties. The hormone has been used in treating amenorrhea, functional uterine bleeding, endometriosis, and for contraception. |
| Cell Research | 96 well plates are seeded with approximately 1000 MCF-7 cells per well in assay kit medium. Subsequently, the cells are incubated with medium containing charcoal/dextran treated serum for three days. The Norethisterone is then added alone to the wells and incubated for seven days. To mimic continuous combined HRT the cells are treated with an oestradiol (0.1 nM)/ Norethisterone combination for seven days. After incubation for seven days, cell proliferation is measured by using an ATP-chemosensitivity test. In brief, proliferation is quantified by measuring light emitted during the bioluminescence reaction of luciferine in the presence of ATP and luciferase.(Only for Reference) |
| In vitro | Norethisterone, or, is a 19-nortestosterone derivative, that lacks a C19 methyl group and possesses C17 ethinyl substitution, and primarily displays progestational activity rather than androgenic activity and, to a lesser extent, has oestrogenic and anti-oestrogenic activity. [1] NET shows five- to eight-fold weaker progesterone receptor binding and transactivation activities than the Org 2058 (100%) and two-fold stronger than progesterone. Binding and transactivation activities of NET for androgen receptor (5α-dihydrotestosterone 100%) are 3.2 and 1.1%, respectively, for estrogen receptor none (estradiol 100%) and for glucocorticoid receptor below 1% (dexamethasone 100%). [2] Norethisterone (1 nM) inhibits serum-stimulated or oestradiol (0.1 nM)-induced proliferation of MCF-7 by 41% and 34%, respectively. [3] Norethisterone (50 nM) induces signi?cant effects on rat osteoblast proliferation, differentiation, and mineralization processes, mimicking the effects of estradiol, which is mediated by estrogen receptor. [4] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | DMSO : 20 mg/mL (67.02 mM), Sonication is recommended.
Ethanol : 5 mg/mL (16.75 mM), Sonication is recommended.
|
| Keywords | progestogenReceptor | progestogen Receptor | ProgesteroneReceptor | Progesterone Receptor | NR3C3 | Norethindrone | Inhibitor | inhibit | EstrogenReceptor | Estrogen Receptor | ER |
| Inhibitors Related | Neomycin sulfate | Adipic dihydrazide | Levulinic acid | D(+)-Raffinose pentahydrate | Sulfamethoxazole sodium | Terbinafine hydrochloride | Metronidazole | Copper(Ⅱ) Sulfate | Doxycycline | Dimethyl sulfoxide | Sodium diacetate | BES |
| Related Compound Libraries | Failed Clinical Trials Compound Library | Bioactive Compound Library | ReFRAME Related Library | Anti-Breast Cancer Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Endocrinology-Hormone Compound Library | NO PAINS Compound Library | FDA-Approved Drug Library | Orally Active Compound Library | Bitter Compound library | Bioactive Compounds Library Max |

 United States
United States