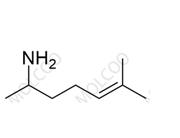
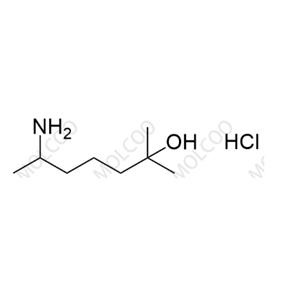
Heptaminol Hydrochloride

Product Code:H012000A
English Name:Heptaminol Hydrochloride
English Alias:6-amino-2-methylheptan-2-ol hydrochloride
CAS No.:543-15-7
Molecular Formula:C₈H₁₉NO·HCl
Molecular Weight:181.70(145.24 + 36.46)
High Purity:Confirmed by HPLC (≥99.5%), combined with multiple techniques such as NMR (1H, 13C), HRMS, and elemental analysis, ensuring stable and reliable product quality to meet high-standard experimental and production requirements.
Good Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.2% in conventional aqueous solution environment within 6 months, guaranteeing the accuracy of experimental data and the stability of production batches.
Raw Material for Cardiovascular Drugs:As a key intermediate for synthesizing cardiovascular drugs, it is used to prepare drugs that enhance myocardial contractility, improve cardiac pumping function, and clinically treat heart failure, cardiogenic shock, and other diseases.
Reference Standard in Pharmaceutical Research:In new drug development, it serves as a standard reference substance to detect the content of related impurities in drugs, helping to establish quality control standards and ensuring the safety and effectiveness of drugs.
Intermediate in Organic Synthesis:In the field of organic synthesis, the reactivity of its amino and hydroxyl groups enables it to be used in constructing various complex compound structures, expanding the synthesis routes of new drugs or functional materials.
Heptaminol Hydrochloride (6-amino-2-methylheptan-2-ol hydrochloride) belongs to the class of amino alcohol compounds. Due to its unique chemical structure and physiological activity, it holds an important position in the field of cardiovascular treatment. This compound enhances myocardial contractility by increasing the calcium ion concentration in myocardial cells, thereby improving cardiac function. With the increase in the incidence of cardiovascular diseases globally, the demand for drugs related to Heptaminol Hydrochloride continues to grow. The optimization of its synthesis process, quality control, and development of new applications have become research hotspots in the pharmaceutical field.
Synthesis Process Optimization:The current mainstream synthesis method employs Grignard reagent reactions combined with reductive amination steps. By optimizing catalysts (such as using novel nanoscale metal catalysts) and reaction conditions (precisely controlling temperature, pressure, and reaction time), the yield can be increased to over 85%, while reducing the occurrence of side reactions.
Quality Detection Technology:Ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) technology, combined with a C18 column and gradient elution conditions, can complete separation within 5 minutes, with a detection limit as low as 0.001 ng/mL, enabling precise detection of trace impurities and ensuring that product quality meets international pharmacopoeia standards.
Clinical Application Expansion:In addition to traditional treatment of heart failure, studies have found that it has potential in improving the recovery of cardiac function after acute myocardial infarction. Relevant clinical trials are underway to explore its broader clinical application value and safety.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China