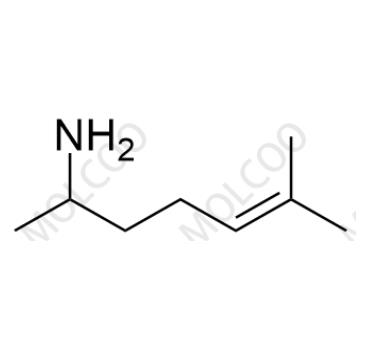
Methyl heptenone
High-Purity Guarantee:Confirmed by HPLC (≥99.0%), combined with multiple techniques such as NMR (1H, 13C), HRMS, and elemental analysis, providing accurate reference materials for Heptaminol Hydrochloride quality control.
Good Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.3% in common organic solvents (such as methanol, acetonitrile) within 6 months, ensuring stable and reliable experimental data.
Quality Control Testing:Used for UPLC-MS/MS detection of EP Impurity A in Heptaminol Hydrochloride API and formulations, strictly controlling impurity content to meet European Pharmacopoeia (EP) and ICH Q3A standards (single impurity limit ≤0.1%).
Process Optimization Research:Monitor the formation pathway of this impurity during Heptaminol Hydrochloride synthesis. Reduce impurity generation by over 50% by adjusting reaction temperature, solvent system, or catalyst type to improve product quality.
Method Validation:Serves as a standard for developing and validating impurity detection methods, ensuring the specificity, sensitivity, and accuracy of the detection methods to meet regulatory requirements for pharmaceutical quality research.
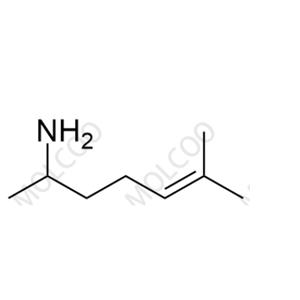
Heptaminol Hydrochloride is a drug used to improve cardiac function. During its production, due to raw material residues, side reactions, or improper control of synthesis process conditions, Impurity A (6-methylhept-5-en-2-amine) may be generated. The presence of this impurity may affect the stability, safety, and efficacy of Heptaminol Hydrochloride. With the increasingly strict requirements of global regulatory agencies for drug impurity control, the study and control of Heptaminol Hydrochloride Impurity A have become a key link in ensuring drug quality and patient safety.
Detection Technology:UPLC-MS/MS with a C18 column (1.7μm) and 0.1% formic acid - acetonitrile gradient elution achieves separation within 3 minutes, with an LOD as low as 0.002 ng/mL, enabling precise detection of trace impurities.
Formation Mechanism:Studies have shown that this impurity may be generated by isomerization, cyclization, and other side reactions of unsaturated amine substances in raw materials under high temperature or strongly alkaline conditions. Its formation can be effectively inhibited by optimizing the reaction temperature (controlled below 50°C) and using mild alkaline reagents.
Safety Evaluation:In vitro cytotoxicity tests show that the IC₅₀ of this impurity against H9c2 cardiomyocytes is 168.5 μM (Heptaminol Hydrochloride IC₅₀ = 12.3 μM). Although less toxic than the main drug, its content in drugs still needs to be strictly controlled. Currently, long-term stability tests are being carried out to systematically monitor its degradation behavior under different humidity, light, and temperature conditions.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China