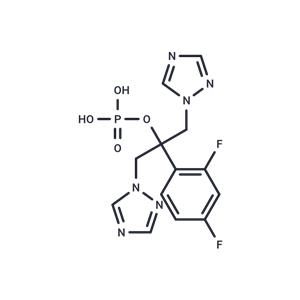
| Name | Fosfluconazole |
| Description | Fosfluconazole is water-soluble phosphate prodrug of fluconazole. Fluconazole is an antifungal drug. |
| Kinase Assay | An aliquot of 200 μl of mucosa scrap lysate solution was mixed with 100 mM phosphate buffer, pH 7.4, to a final volume at 1 ml. The concentration of the test compounds (fosphenytoin and fosfluconazole) was 10 μM. The incubation medium was prewarmed at 37°C before the reaction was initiated by addition of the tested compounds. An aliquot of 100 μl was collected from the incubation vial at the time points 0, 5, 10, 20, 30, 45, and 60 min and transferred to a 96-well plate, in which 100 μl of acetonitrile was prefilled to terminate the reaction. The samples were diluted 5-fold with acetonitrile containing 1 μM tolbutamide as an analytical internal standard. The samples were centrifuged at 4000 rpm for 5 min to precipitate protein. The supernatant was transferred to a new 96-well plate for concentration analysis by liquid chromatography/tandem mass spectrometry (LC/MS/MS) [1]. |
| Animal Research | Twelve-week-old male Wistar rats (200–300 g) were used. They were housed in a temperature-controlled room and were given food and water ad libitum. All rats were anesthetized by an i.p. administration of pentobarbital (50 mg/kg body weight). A catheter was placed in the peritoneal cavity and used as an inflow drain for the dialyzing fluid. sampling. In rats receiving i.v. administration, a catheter was inserted into the left jugular vein for drug administration, and another into the right femoral artery for blood sampling. Blood samples after the i.p. administration were obtained from the heart. The rats were then allowed to recover from the anesthesia and surgery for at least 24 hr. After the 40-mL dialyzing fluid was administered intraperitoneally, FLCZ 16 mg/kg body weight) and F-FLCZ (16 mg FLCZ eq/kg body weight) were administered intravenously. The volume of the drug solution administered intravenously was 8 mL/kg. FLCZ (16 mg/kg body weight) and F-FLCZ (16 mg FLCZ eq/kg body weight) dissolved in the 40 mL of dialyzing fluid were administered intraperitoneally. The dialyzing fluid FLCZ concentration was 100 mg/L. F-FLCZ (16 mg FLCZ eq/kg body weight) was administered to ARF rats intravenously and intraperitoneally. Blood (0.5 mL) and dialyzing ‰uid (1.5 mL) samples were collected at appropriate time intervals [2]. |
| In vitro | In Caco-2 monolayer, 10 μM Fosfluconazole is dosed either in the apical or basal compartment in Transwell plates. Both prodrugs are efficiently cleaved in the apical compartment after a 2 h incubation. The rate of ALP-mediated conversion was prodrug concentration-dependent with Michaelis-Menten constants of 351 μM for fosfluconazole, determined in Caco-2 cells [1]. |
| In vivo | Fosfluconazole was administered intravenously and intraperitoneally. After the i.p. administration of F-FLCZ, FLCZ was detected in circulating blood and the dialyzing fluid in peritoneal dialysis rats. The concentration of plasma FLCZ after the i.p. F-FLCZ administration was lower than that after the intravenous (i.v.) F-FLCZ administration [2]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 6 mg/mL (15.53 mM)
|
| Keywords | Fungal | Inhibitor | inhibit | Fosfluconazole |
| Inhibitors Related | Dehydroacetic acid sodium | 2-Butyl-1,2-benzisothiazolin-3-one | Tebuconazole | Veratraldehyde | Paclobutrazol | Lauryl betaine | Ammonium Chloride | Potassium sorbate | Geraniol | Sorbic acid |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Bioactive Compound Library | Approved Drug Library | ReFRAME Related Library | Anti-Fungal Compound Library | Drug Repurposing Compound Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | Fluorochemical Library | Human Metabolite Library |

 United States
United States