Factory Provide High Quality Anti -Depression Fluoxetine CAS 54910-89-3

Introduction
Product Name :Fluoxetine
Other name:(+/-)-n-methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanamine;METHYL-[3-PHENYL-3-(4-TRIFLUOROMETHYLPHENOXY)PROPYL]AMINE
CAS No.: 54910-89-3
MF:C17H18F3NO
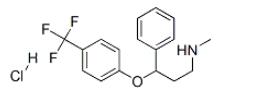
MW:309.33
Appearance: White Powder
Melting Point :158 °C (dec.)(lit.)
Boiling Point :395.1±42.0 °C(Predicted)
Density :1.159±0.06 g/cm3(Predicted)
Storage :Keep in dark place,Sealed in dry,Room Temperature
Fluoxetine was shown to be effective for depression in six-week-long double-blind controlled trials, where it also alleviated anxiety and improved sleep. Fluoxetine was better than placebo for the prevention of depression recurrence when the patients, who originally responded to fluoxetine, were treated for a further 38 weeks. Efficacy of fluoxetine for geriatric, as well as pediatric, depression was also demonstrated in placebo-controlled trials.
Research suggests that a significant part of the resistance to the SSRIs paroxetine (Paxil) and citalopram(Celexa) can be explained by the genetic variation of Pgp transporter. Paroxetine and citalopram, which are Pgp substrates, are actively transported from the brain by this protein. Fluoxetine is not a substrate of Pgp, and thus a switch from paroxetine or citalopram to fluoxetine may be beneficial to the nonresponders.
Function & Application
Medical uses:
Fluoxetine 20mg blister pack
Fluoxetine is frequently used to treat major depressive disorder, obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), bulimia nervosa, panic disorder, premenstrual dysphoric disorder, and trichotillomania.It has also been used for cataplexy, obesity, and alcohol dependence,as well as binge eating disorder.It has also been tried as a treatment for autism spectrum disorders with moderate success in adults.
Obsessive-compulsive disorder:
The efficacy of fluoxetine in the treatment of obsessive-compulsive disorder (OCD) was demonstrated in two randomized multicenter phase III clinical trials. The pooled results of these trials demonstrated that 47% of completers treated with the highest dose were "much improved" or "very much improved" after 13 weeks of treatment, compared to 11% in the placebo arm of the trial.The American Academy of Child and Adolescent Psychiatry state that SSRIs, including fluoxetine, should be used as first-line therapy in children, along with cognitive behavioral therapy (CBT), for the treatment of moderate to severe OCD.
Panic disorder:
The efficacy of fluoxetine in the treatment of panic disorder was demonstrated in two 12-week randomized multicenter phase III clinical trials that enrolled patients diagnosed with panic disorder, with or without agoraphobia. In the first trial, 42% of subjects in the fluoxetine-treated arm were free of panic attacks at the end of the study, vs. 28% in the placebo arm. In the second trial, 62% of fluoxetine treated patients were free of panic attacks at the end of the study, vs. 44% in the placebo arm.
Bulimia nervosa:
A 2011 systematic review of seven trials which compared fluoxetine to a placebo in the treatment of bulimia nervosa; six of which found a statistically significant reduction in symptoms such as vomiting and binge eating.However, no difference was observed between treatment arms when fluoxetine and psychotherapy were compared to psychotherapy alone.


 China
China