Factory Provide CAS 59333-67-4 Fluoxetine Hydrochloride / Fluoxetine HCl

Introduction
Product Name :Fluoxetine Hydrochloride; Fluoxetine HCl
Other name:SARAFEM;Affectine;Alzac 20;N-Methyl-3-phenyl-3-(4-(trifluoromethyl)phenoxy)propylamine hydrochloride
CAS No.: 59333-67-4
MF:C17H19ClF3NO
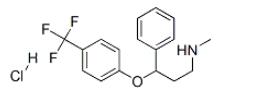
MW:345.79
Appearance: White Powder
Melting Point :158-159°C (dec.)(lit.)
Storage :Keep in dark place,Sealed in dry,Room Temperature
Fluoxetine hydrochloride discovery of fluoxetine began at Eli Lilly in 1970 as a collaboration between Bryan Molloy and Robert Rathbun. Testing the physiological effects of these compounds in mice resulted in nisoxetine, a selective norepinephrine reuptake inhibitor currently widely used in biochemical experiments. This test which carried out by Jong-Sir Horng in May 1972, showed the compound later named fluoxetine to be the most strong and selective inhibitor of serotonin reuptake of the series. Two years later they had to issue a correction, admitting that the first SSRI was zimelidine developed by Arvid Carlsson and colleagues. In 1986, Fluoxetine made its appearance on the Belgian market and was approved for use by the FDA in the United States in December 1987. Fluoxetine was the fourth SSRI to make it to market, after zimelidine, indalpine and fluvoxamine.
Function & Application
Medical uses:
Fluoxetine 20mg blister pack
Fluoxetine is frequently used to treat major depressive disorder, obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), bulimia nervosa, panic disorder, premenstrual dysphoric disorder, and trichotillomania.It has also been used for cataplexy, obesity, and alcohol dependence,as well as binge eating disorder.It has also been tried as a treatment for autism spectrum disorders with moderate success in adults.
Obsessive-compulsive disorder:
The efficacy of fluoxetine in the treatment of obsessive-compulsive disorder (OCD) was demonstrated in two randomized multicenter phase III clinical trials. The pooled results of these trials demonstrated that 47% of completers treated with the highest dose were "much improved" or "very much improved" after 13 weeks of treatment, compared to 11% in the placebo arm of the trial.The American Academy of Child and Adolescent Psychiatry state that SSRIs, including fluoxetine, should be used as first-line therapy in children, along with cognitive behavioral therapy (CBT), for the treatment of moderate to severe OCD.
Panic disorder:
The efficacy of fluoxetine in the treatment of panic disorder was demonstrated in two 12-week randomized multicenter phase III clinical trials that enrolled patients diagnosed with panic disorder, with or without agoraphobia. In the first trial, 42% of subjects in the fluoxetine-treated arm were free of panic attacks at the end of the study, vs. 28% in the placebo arm. In the second trial, 62% of fluoxetine treated patients were free of panic attacks at the end of the study, vs. 44% in the placebo arm.
Bulimia nervosa:
A 2011 systematic review of seven trials which compared fluoxetine to a placebo in the treatment of bulimia nervosa; six of which found a statistically significant reduction in symptoms such as vomiting and binge eating.However, no difference was observed between treatment arms when fluoxetine and psychotherapy were compared to psychotherapy alone.







 China
China




