Manufacturer Provide Veterinary Medicine Enrofloxacin hydrochloride Powder CAS 112732-17-9 Enrofloxacin HCl

Introduction
1.Product Name:Enrofloxacin Hcl/Enrofloxacin hydrochloride
2.Synonyms:3-Quinolinecarboxylicacid, 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-,hydrochloride (9CI);Enrofloxacin hydrochloride;
3.CAS NO: 112732-17-9
4.Purity:99%
5.Appearance:White Crystalline Powder
6.Molecular formula: C19H23ClFN3O3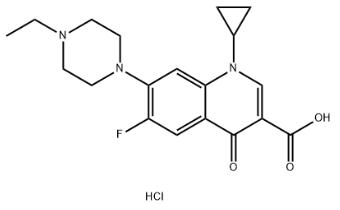
7.Molecular weight :395.86
8.Melting Point :>285°C (dec.)
9.Solubility : Soluble in methanol (slightly heated), water (slightly heated)
10.Storage :Keep in a cool,dry,dark location in a tightly sealed container or cylinder.
Application
1. Enoxacin hydrochloride is the third generation of quinolone antibacterial drug, also known as ethylciprofloxacin and enfloxacin.Approved by FDA on October 4, 1996, quinolones for livestock, poultry and aquatic products.
2. Broad-spectrum antibacterial activity and strong permeability. This product has strong killing effect on gram-negative bacteria and good antibacterial effect on gram-positive bacteria and mycoplasma.Can significantly reduce the death rate, disease animal recovery, rapid growth.
3. Unique and direct bactericidal method, directly acting on the bacterial nucleus, inhibiting bacterial DNA rotase, leading to rapid death of bacteria and not easy to produce drug resistance.No cross-resistance with other antibiotics.
4, the effect is rapid, 1 hour after the drug will play a role.
5. High safety. The difference between the amount of treatment and the amount of poisoning is 250 times.
| Description | White powder | Complies |
| Heavy metal | ≤10ppm | 5ppm |
| Pb | ≤3ppm | 1.5ppm |
| Hg | ≤0.1ppm | 0.05ppm |
| Cd | ≤1ppm | 0.2ppm |
| Loss on drying | ≤0.5% | 0.12 |
| Residue on lgnition | ≤0.1% | 0.03 |
| Single impurity | ≤0.5% | 0.12 |
| Total impurity | ≤1.0% | 0.29 |
| Assay | ≥99.0% | 99.4% |







 China
China




