Manufacturer Provide Antibiotic Medicine Antibacterial Drug CAS93106-60-6 Enrofloxacin

Introduction
1.Product Name: Enrofloxacin
2.CAS NO: 93106-60-6
3.Purity: 99%
4.Appearance:Slightly Yellow Crystalline Powder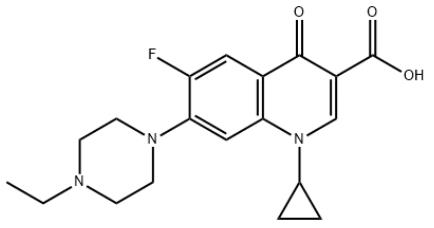
5.Molecular formula: C19H22FN3O3
6.Molecular weight: 359.39
7.Melting Point :359.39 (lit.)
8.Solubility: Soluble in chloroform. Slightly soluble in water. Also soluble in dilute KOH
Application:
1. Antimicrobial, for bacteria and mycoplasma infection. Fluorinated quinolone antibacterial
2. New veterinary antimicrobial drugs, broad-spectrum, high efficiency, has special effects on Gram-positive and Gram-negative bacteria and mycoplasma.
Precautions:
1. Antacids can inhibit the absorption of this product, should be avoided drinking at the same time
2. In clinical application, can appropriately adjust dosage based on disease, concentration range of drinking water in poultry, per liter of water, added 25 to 100 mg.
3. Withdrawal period of chicken is 8 days. Disabled in egg producing period of laying hen.
| TEST REPORT |
| ITEM | SPE | RESULT |
| Appearance | Slightly yellow or pale orange yellow crystalline powder | Slightly yellow crystalline powder |
| ldentification | Meet the requirements | Meet the requirements |
| Melting range | 221~-226C | 223.5-224.5C |
| Heavy metals | ≤20ppm | <20ppm |
| Loss on drying | ≤0.5% | 0.07 % |
| Residue on ignition | ≤0.2% | 0.03% |
| Related substances | Ciprofoxacin ≤0.5% | 0.14% |
| Any otherr single impurity ≤0.3% | 0.14% |
| Total impuries ≤ 0.7% | 0.14% |
| Fluoroquinolonie acid (TLC) | ≤0.2% | <0.2% |
| Assay | ≥99.0% (Drled substasce) | 100.1% |
| Concluslon | Conforms to Chinese Veterinary Pharmacopoeis2015 ( I ) specifcation for carofioxacin |








 China
China




