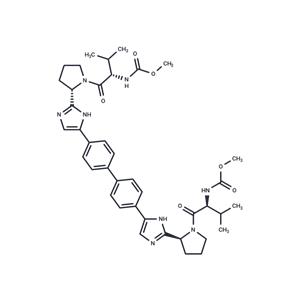
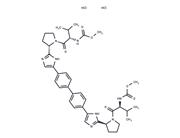
| Name | Daclatasvir |
| Description | Daclatasvir (EBP 883) (BMS-790052) is a highly selective inhibitor of HCV NS5A with EC50 of 9-50 pM, for a broad range of HCV replicon genotypes and the JFH-1 genotype 2a infectious virus in cell culture. Phase 3. |
| Cell Research | BMS-790052 is added to 96-well plates containing HCV replicon cells seeded approximately 12?hours before in 200 μL media.The cell plates are tested for replication activity and cytotoxicity after 72 hours of incubation. Cytotoxicity is measured with CellTiter-Blue, after which the media and dye are removed, plates are inverted and the remaining liquid is blotted with paper towels. Replication activity of the HCV genotype 1a cell lines is quantified using Renilla luciferase. 1× Renilla luciferase lysis buffer (30 μL) is added to each well and plates are incubated with gentle shaking for 15?min. Renilla luciferase substrate (40 μL) is then added and the signals are detected using a Top Count luminometer set for light emission quantification. One hundred per cent activity is calculated for each cell line for the DMSO-only wells; percentage activity is calculated for each concentration of the inhibitor by dividing the average value for wells containing compound by the average value for wells containing DMSO.(Only for Reference) |
| Kinase Assay | FRET assay for HCV NS5A inhibitors: The peptide (Ac-Asp-Glu-Asp [EDANS]-Glu-Glu-Abu-[COO] Ala-Ser-Lys [DABCYL]-NH2) contains a fluorescence donor {EDANS, 5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid} near one end of the peptide and an acceptor {DABCYL, 4-[(4-dimethylamino)phenyl]azo)benzoic acid} near the other end. Intermolecular resonance energy transfer between the donor and the acceptor quenches the fluorescence of the peptide, but as the NS3 protease cleaves the peptide, the products are released from resonance energy transfer quenching. The fluorescence of the donor increases over time as more substrate is cleaved by the NS3 protease. The assay reagent is: 5× luciferase cell culture lysis reagent diluted to 1× with dWater, NaCl (150 mM), the FRET peptide (20 μM). HCV-Huh-7 cells are placed in a 96-well plate, and allowed to attach overnight (1×104 cells per well). The next day, BMS-790052 is added to the wells and the plate is incubated for 72 hours. The plate is then rinsed with PBS and used for the FRET assay by the addition of 30 μL of the FRET peptide assay reagent (described above) per well. Signals are obtained using the Cytofluor 4000 instrument, which has been set to 340 nm (excitation)/490 nm (emission) automatic mode, for 20 cycles or less, with the plate being read in the kinetic mode. Following FRET, 40 μL of luciferase substrate is added to each well and the luciferase is measured. |
| In vitro | Daclatasvir is one of the most potent inhibitors of HCV replication reported so far. The mean EC50 valuses of Daclatasvir are 50 and 9pM for HCV genotype 1a and 1b replicons, respectively. Daclatasvir displays a therapeutic index (CC50/EC50) of at least 105 and is inactive towards a panel of 10 RNA and DNA viruses, with EC50 higher than 10μM. This confirms Daclatasvir's specificity for HCV. [1] In Huh7 cells harboring the HCV genotype 1b replicons, Daclatasvir blocks both transient and stable HCV genome replication, with EC50 values raging from 1-15 pM. Daclatasvir (100 pM or 1 nM) has been shown to alter the subcellular localization and biochemical fractionation of NS5A. [2] Daclatasvir inhibits hybrid replicons containing HCV genotype-4 NS5A genes with EC50 of 7-13 pM. Residue 30 of NS5A is an important site for Daclatasvir-mediated resistance in the hybrid replicons. [3] |
| In vivo | In a randomized, double-blind, placebo-controlled, single ascending-dose study, Daclatasvir (BMS-790052) is administered at six dose levels to healthy, non-HCV-infected subjects over a range of 1 to 200?mg as an oral solution. Daclatasvir is safe and well tolerated up to 200?mg with no clinically relevant adverse effects. After oral administration, Daclatasvir is readily absorbed, with dose-proportional exposures over the studied dose range, and all subjects have drug concentrations greater than the protein-binding-adjusted EC90 for genotypes 1a and 1b, as measured in the replicon assay, at and beyond 24?h post-dose. (The protein binding-adjusted EC90 figures are derived from an analysis of the effect of the addition of human serum on antiviral activity in replicons. In the presence of 40% human serum, the EC90 for Daclatasvir is 383?pM (0.28?ng/mL) for the genotype 1a replicon and 49?pM (0.04?ng/mL) for the genotyope 1b replicon)[1]. Mice in each group that developed persistent HCV infection are divided into two treatment groups. One group receive 4 weeks of Asunaprevir/Daclatasvir treatment and the other group received 4 weeks of Ledipasvir/GS-558093 treatment. Asunaprevir/Daclatasvir therapy and Ledipasvir/GS-558093 therapy rapidly decease serum HCV RNA levels to below the sensitivity, and they are not detected after completion of the therapy except for two mice in the Ledipasvir/GS-558093 group[5]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : < 1 mg/mL (insoluble or slightly soluble)
DMSO : 136 mg/mL (184.1 mM)
Ethanol : 136 mg/mL (184.1 mM)
|
| Keywords | antiviral | Inhibitor | genotypes | EBP883 | EBP-883 | BMS790052 | NS5A | OATP1B1 | Hepatitis C virus | OATP1B3 | HCV | replicon | BMS 790052 | Daclatasvir | inhibit | JFH-1 |
| Inhibitors Related | EIDD-1931 | RO8191 | Ribavirin | Sofosbuvir | Deferiprone | Ombitasvir | Artemisinin | HCV-IN-29 | Honokiol | Resiquimod |
| Related Compound Libraries | Highly Selective Inhibitor Library | Bioactive Compound Library | Approved Drug Library | EMA Approved Drug Library | Drug Repurposing Compound Library | Anti-Viral Compound Library | Inhibitor Library | FDA-Approved Drug Library | Clinical Compound Library | Bioactive Compounds Library Max |

 United States
United States