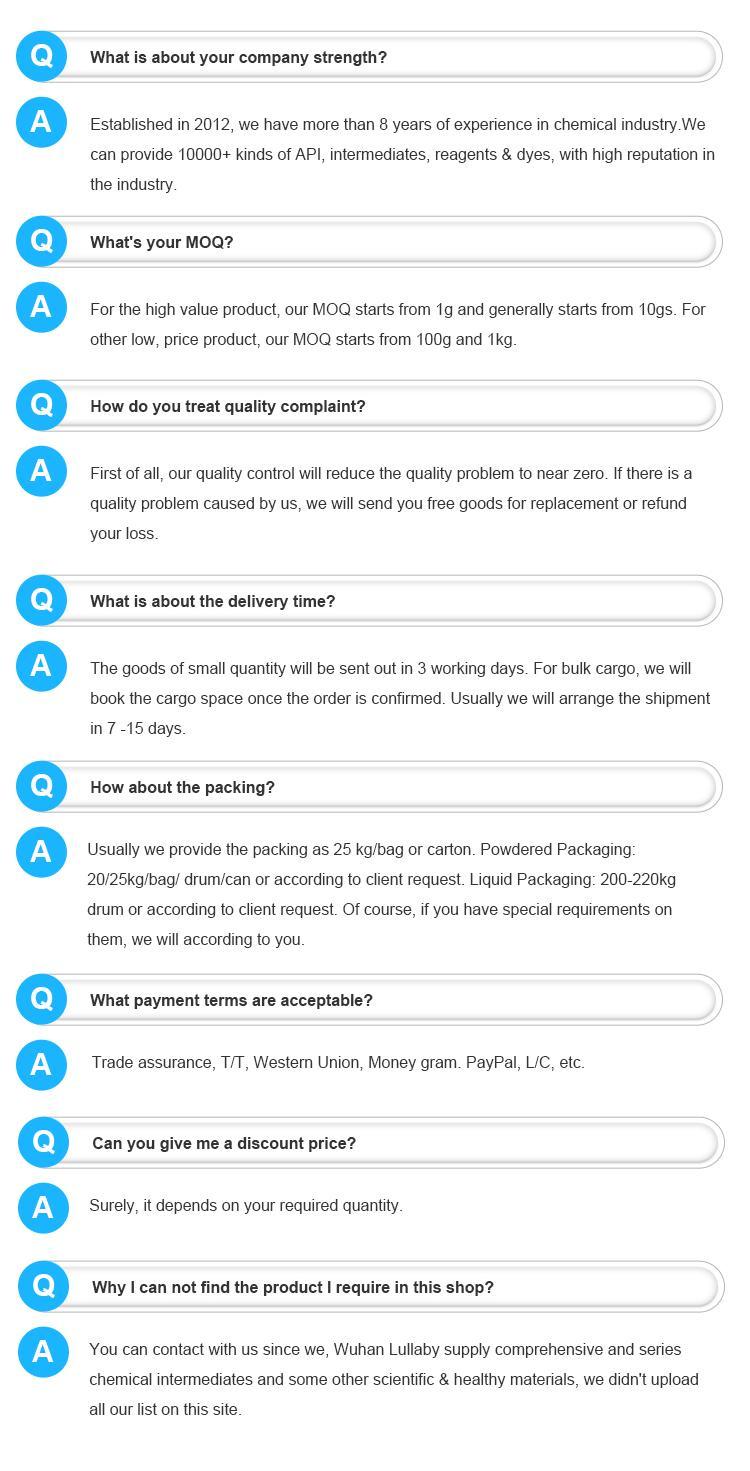
Copper(II) hydroxide has been known since copper smelting began around 5000 BCE although the alchemists were probably the first to manufacture it. This was easily done by mixing solutions of lye and blue vitriol, both chemicals which were known in antiquity.
It was produced on an industrial scale during the 17th and 18th centuries for use in pigments such as blue verditer and Bremen green. These pigments were used in ceramics and painting.
| Name | Cupric hydroxide | EINECS | 243-815-9 |
| CAS No. | 20427-59-2 | Density | 3.37 g/cm3 |
| PSA | 40.46000 | LogP | -0.35610 |
| Solubility | N/A | Melting Point | 160oC
|
| Formula | Cu.(OH)2 | Boiling Point | 100 °C at 760 mmHg |
| Molecular Weight | 97.5607 | Flash Point | N/A |
| Transport Information | UN 3262 | Appearance | Blue powder |
The Copper hydroxide is an organic compound with the formula Cu.(OH)2. The IUPAC name of this chemical is copper dihydroxide. With the CAS registry number 20427-59-2, it is also named as Kupfer(2+)dihydroxid. The product's category is Inorganics. Besides, it is a blue powder, which should be stored at temperature of 0 - 6 °C. It is used as raw material of copper, mordant, rayon, paint, and also used as a fungicide and anti-fouling paint composition.
Appearance and properties: blue or blue-green solid
Density: 3.37g /cm3
Boiling point: 100ºC at 760 mmHg
Melting point: 160ºC
Stability: Stable, but hygroscopic. Store in dry conditions.
Storage conditions: the warehouse is ventilated and dry at low temperature, and stored and transported separately from food raw materials
Usage:Copper hydroxide [Cu(OH)2], slightly toxic, used as an analytical reagent, but also used in medicine, pesticides and so on. It can be used as catalyst, mordant, pigment, feed additive, paper dyeing agent, etc.







 China
China



