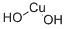| Description |
Cupric hydroxide appears as a blue powder and it is not stable. Cupric hydroxide is used as a mordant and pigment, in the manufacture of many copper salts, and for staining paper. It is used as a fungicide/bactericide on fruits, vegetables, and ornamentals. It can be used as catalyst, feed additive, and a cuprammonium rayon process reagent to make the first semi-synthetic fiber product, Rayon. Furthermore, cupric hydroxide is used in the wood preservative products in aqueous systems. |
| References |
[1] Michael Ash, Handbook of Preservatives, 2004
[2] Günter Joseph and Konrad J. A. Kundig, Copper: Its Trade, Manufacture, Use, and Environmental Status, 1999
[4] Bo Liu, Bin Liu, Yongbo Zhou and Wanzhi Chen, Copper(II) Hydroxide Complexes of N-Heterocyclic Carbenes and Catalytic Oxidative Amination of Arylboronic Acids, Organnometallics, 2010, vol. 29, 1457-1464
[5] George B. Kauffman, Rayon: The first semi-synthetic fiber product, Journal of Chemical Education, 1993, vol. 70, 887 |
| Chemical Properties |
Blue powder.Soluble in acids and ammonium hydroxide; insoluble in water. |
| Chemical Properties |
Blue, gelatinous or amorphous powder. Insoluble in water. |
| Uses |
In manufacture of rayon, battery electrodes, other Cu salts; as mordant in dyeing; as pigment; in fungicides, insecticides; as feed additive; in treating and staining paper; in preparation of Schweitzer's reagent; in catalysts. |
| Hazard |
Toxic by ingestion and inhalation. |
| Potential Exposure |
Inorganic copper fungicide, nematicide, and microbiocide |
| First aid |
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions) if breathing has stopped, and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious and able to swallow, have victim drink 4 to 8 oz of water. Do not induce vomiting. |
| Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
| Incompatibilities |
Reacts with calcium (metal hydroxides), nitroethane, nitromethane, 1-nitropropane, zirconium |
| Waste Disposal |
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Copper-containing wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. Details of copper recovery from a variety of industrial wastes have been published. If recovery is not feasible, the copper can be precipitated by the use of caustics and the sludge deposited in a chemical waste landfill. Recommendable methods: Precipitation, solidification, landfill, discharge to sewer, & incineration. Peer-review: Precipitate copper with alkali, filter, solidify precipitate. (Do not use ammonia as alkali). Cation exchange will allow recovery of copper. Eluate from cation exchanger can be passed through anion exchanger to remove (or reduce) naphthenic acid content. Exhausted ion exchange resins can be landfilled. (Peer-review conclusions of an IRPTC expert consultation) |

 China
China