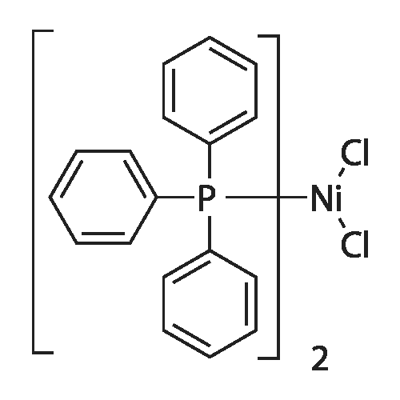
| CAS: | 14264-16-5 |
| MF: | C36H30Cl2NiP2 |
| MW: | 654.17 |
| EINECS: | 238-154-8 |
| Product Categories: | Metal Compounds;Catalysts for Organic Synthesis;Classes of Metal Compounds;Homogeneous Catalysts;Metal Complexes;Ni (Nickel) Compounds;Synthetic Organic Chemistry;Transition Metal Compounds;metal-phosphine complexes;Aromatics;Phosphorylating and Phosphitylating Agents;Pyridines;Ni |
| Mol File: | 14264-16-5.mol |
 |
|
| Bis(triphenylphosphine)nickel(II)chloride Chemical Properties |
| Melting point | 250 °C (dec.)(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Crystals or Powder |
| color | Dark green to dark gray |
| Water Solubility | insoluble |
| Sensitive | Hygroscopic |
| Exposure limits | NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| CAS DataBase Reference | 14264-16-5(CAS DataBase Reference) |
| NIST Chemistry Reference | dichlorobis(triphenylphosphine)nickel(14264-16-5) |
| EPA Substance Registry System | Nickel, dichlorobis(triphenylphosphine)- (14264-16-5) |
| Bis(triphenylphosphine)nickel(II)chloride Usage And Synthesis |
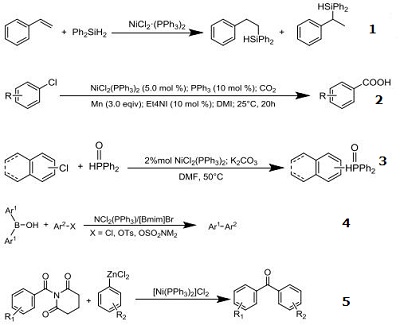
| Reaction | Catalyst for hydrosilylation of styrene with diphenysilane Catalyst for carboxylation of various aryl chlorides and other derivatives Catalyst for C–P cross-coupling reactions of diphenylphosphine oxide with aryl chloride Catalyst for N?Heterocyclic carbene-assisted cross-coupling reactions of diarylborinic acids with aryl chlorides,tosylates, and sulfamates. Catalyst for Negishi biaryl ketone synthesis by cross-coupling of amides with aryl zinc halides via carbon-nitrogen bond cleavage.
 |
| Chemical Properties | dark green to dark grey crystals or powder |
| Uses | Coordination compund. |
| Uses | suzuki reaction |
| Uses | Dichlorobis(triphenylphosphine)nickel(II) is used as a catalyst for cross-coupling of Grignard reagents, hydrosilylations, hydrogenation and polymerization. |
| Purification Methods | Wash it with glacial AcOH and dry it in a vacuum over H2SO4 and KOH until AcOH is removed. [Venanzi J Chem Soc 719 1958, Kocienski et al. J Org Chem 54 1215 1989, Beilstein 16 IV 953.] |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock


 Japan
Japan









 Company information
Company information Advantage
Advantage


