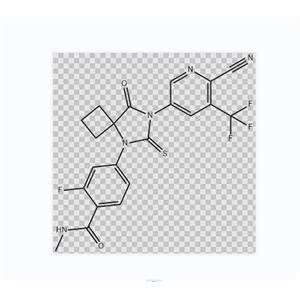
Apalutamide is an androgen receptor (AR) inhibitor developed by the University of California in the United States. The drug was licensed to Aragon Pharmaceuticals in 2009. In February 2018, Apalumide was approved by the US FDA for use in the treatment of non metastatic castration resistant prostate cancer (NM-CRPC). Apalumide belongs to the second generation of highly selective androgen receptor antagonists, and its affinity with androgen receptors is more than five times that of the first generation of androgen receptor antagonists. A phase III study involving 1207 patients with non metastatic castration resistant prostate cancer demonstrated that apalumide significantly prolonged Chemicalbook's metastasis free survival (40.5 vs 16.2 months) compared to placebo. On February 14, 2018, Apalumide was approved by the FDA for use in the treatment of non metastatic (non proliferative prostate cancer cells) castration resistant (disease progression after hormone therapy) prostate cancer. On September 6, 2019, Apalumide was officially approved for marketing by the National Drug Administration, becoming the first drug in China to treat non metastatic castration resistant prostate cancer. Due to the fact that Abitron is only used to treat metastatic castration and resist prostate cancer, and the generic drugs of Hengrui and Zhengda Tianqing Abitron have been launched in China, Apalumide will provide new impetus for Johnson&Johnson's prostate cancer business.

 China
China