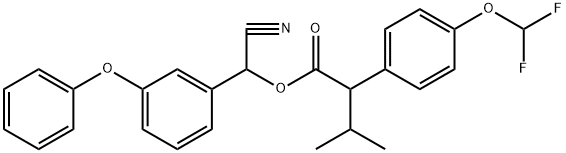
Uses
Nonsystemic insecticide.
Uses
Flucythrinate controls a wide range of insects on cotton, fruit trees, vines, citrus, bananas, pineapples, olives, coffee, cocoa, hops, vegetables, soyabeans, cereals, alfalfa, sugar beet, sunflowers and tobacco.
Agricultural Uses
Insecticide, Acaricide: Not approved for use in EU countries. Not currently registered in the U.S. Flucythrinate is a synthetic pyrethroid used to control pests in apples, cabbage, head lettuce, pears, corn and cotton, but it was used primarily on cotton.
Trade name
AASTAR®[C]; CYBOLT®; CYTHRIN®; FUCHING JUJR®; GUARDIAN®[C]; PAYOFF®[C]; STOCK GUARD®; TOMAHAWK®
Environmental Fate
Surface Water. The half-life of ?ucythrinate in an estuarine environment is 34 days (Schimmel et al., 1983).
Chemical/Physical. Hydrolyzes in aqueous solutions forming acetic acid and other compounds.
Metabolic pathway
Flucythrinate is an analogue of fenvalerate and would be expected to have a similar fate.
Degradation
Flucythrinate is a stable compound but it is readily hydrolysed at alkaline pH to afford 2-(4-difluoromethoxyphenyl)-3-methylbutyric acid (2), 3PBAl(3) and cyanide ion. Its DT50 values at 27 °C are 52 days (pH 5) and 6.3 days (pH 9). It undergoes aqueous and soil surface photolysis with DT50 values of 1-4 days and <2 days, respectively. The products formed under aqueous conditions were 2 and 3. Compound 3 was oxidised to 3PBA (4) and reduced to 3PBAlc (5). The α-carbamoyl (amide) derivative (6) of 3PBAlc was also detected. On soil surfaces, products were similar with the exceptions that 3PBA was not formed and the α-amide (7) and a-carboxy derivative (8) were formed. These are usually considered to be products of thermal degradation (Dureja and Chattopadhyay, 1995).

 China
China