| Description |
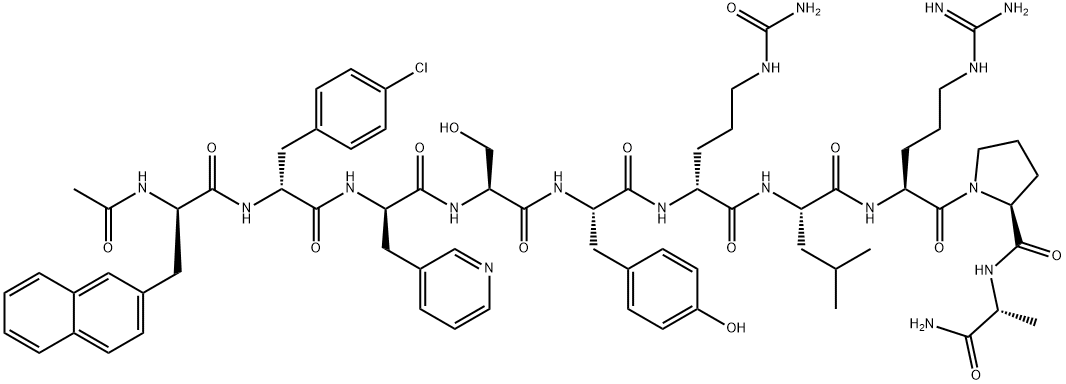
Cetrorelix was launched in Germany for the treatment of female infertility. It is a decapeptidic analog of luteinizing hormone-releasing hormone (LH-RH) bearing structural modifications in the crucial positions 1, 2, 3, 6 and 10 : [Ac-D-Nal1, D-4-CI Phe-2, D-Pal3, D-Cit6, D-Alal0]-GnRH. Cetrorelix is an extremely potent and long acting gonadotrophin releasing hormone (GnRH) antagonist and thus blocks gonadotrophins and sex steroid secretion immediately after administration. Moreover, it has a low histamine-releasing potency. Cetrorelix will be the first LH-RH antagonist approved worldwide. In several Phase III clinical trials, female patients receiving Cetrorelix had a successful controlled ovulation thus avoiding a premature LH-surge. Cetrorelix could be a first-choice in-vitro fertilization (IVF) treatment without the complications of current controlled ovarian hyperstimulation protocols. Cetrorelix is currently under clinical investigation for the treatment of diverse sex hormone dependent disorders such as benign prostate hypertrophy, breast, ovarian or prostate cancers and diverse gynaecological disorders. |
| Definition |
ChEBI: A synthetic ten-membered oligopeptide comprising N-acetyl-3-(naphthalen-2-yl)-D-alanyl, 4-chloro-D-phenylalanyl, 3-(pyridin-3-yl)-D-alanyl, L-seryl, L-t rosyl, N5-carbamoyl-D-ornithyl, L-leucyl, L-arginyl, L-prolyl, and D-alaninamide residues coupled in sequence. A gonadotrophin-releasing hormone (GnRH antagonist, it is used for treatment of infertility and of hormone-sensitive cancers of the prostate and breast. |

 China
China