1/1
Thalidomide
$ 1.00
/1kg
- Min. Order1g
- Purity99%
- Cas No50-35-1
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Thalidomide |
| CAS No | 50-35-1 |
| EC-No | |
| Min. Order | 1g |
| Purity | 99% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
| Thalidomide Basic information |
| Glutamic acid derivatives Treatment of rheumatism Side effects Chemical Properties Uses |
| Product Name: | Thalidomide |
| Synonyms: | (+-)-THALIDOMIDE >98% IMMUNOSUPPRESSIVE, SE;α-Phthalimidoglutarimide;N-PHTHALOLYLGLUTAMIMIDE;2,6-Dioxo-3-phthalimidoglutarimide;a-Phthalimidoglutarimide;n-(2,6-Dixo-3-piperidyl)phthalimide;ThalidomideUsp27;Zeniquine |
| CAS: | 50-35-1 |
| MF: | C13H10N2O4 |
| MW: | 258.23 |
| EINECS: | 200-031-1 |
| Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;API's;Cytokine signaling;Signalling;Influenza Viruses;Angiogenesis and Metastasis;Inhibitor;ROCCAL;API |
| Mol File: | 50-35-1.mol |
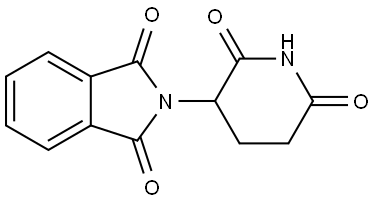 |
|
| Thalidomide Chemical Properties |
| Melting point | 269-271°C |
| Boiling point | 401.48°C (rough estimate) |
| density | 1.2944 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Store at RT |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.6 mg/mL |
| color | white |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| λmax | 300nm(lit.) |
| Merck | 14,9255 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | UEJJHQNACJXSKW-UHFFFAOYSA-N |
| CAS DataBase Reference | 50-35-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Phthalimide, n-(2,6-dioxo-3-piperidyl)-(50-35-1) |
| EPA Substance Registry System | 1H-Isoindole-1,3( 2H)-dione, 2-(2,6-dioxo-3-piperidinyl)- (50-35-1) |
| Safety Information |
| Hazard Codes | T |
| Risk Statements | 46-61-21-25-62-22 |
| Safety Statements | 53-22-26-36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | TI4375000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Hazardous Substances Data | 50-35-1(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| 2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione | English |
| SigmaAldrich | English |
| Thalidomide Usage And Synthesis |
| Glutamic acid derivatives | Thalidomide is a kind of synthetic glutamic acid derivatives. At room temperature, it is a kind of white crystalline powder, and is odorless, tasteless, and slightly soluble in water, methanol, ethanol or acetone, highly soluble in dimethylformamide or pyridine, but insoluble in ether, chloroform or benzene. At 1950s, Germany developed the drug mainly for treating epilepsy. However, due to the lack of efficacy, then it is further used as an adjunct for sleeping while also widely used as antiemetic drug for pregnant women during their pregnancy. At early 1960s, thalidomide incident---there had been a lot of reports about birth defect caused by thalidomide (such as: short limb malformations, bone defect, ear missing, cleft lip, heart and gastrointestinal tract abnormalities, etc.). Thereby, it was further prohibited by many countries, and subjected to withdrawal from the pharmaceutical market; but scientists did not totally negate thalidomide and continued to carry out in-depth research; there has been much encouraging and promising progress on the pharmacologic mechanisms of immunity, anti-inflammatory, and anti-angiogenic as well as the clinical treatment of various kinds of difficult disease, making people gain new understanding of the functions of thalidomide. Since the 1970s, with the emergence of the various research progresses of leprosy, rheumatism and various types of cancer, Israel dermatologists had applied thalidomide as a sedative for patients of erythema nodosum leprosy and obtain rapid alleviation of symptoms. After that many patients of erythema nodosum leprosy had received good therapeutic effects. In1998, the FDA approved thalidomide for the treatment of erythema nodosum leprosy. In 2004, during the American Society of Hematology annual meeting, RaJkumar from the US Mayo Clinic reported the progress of two studies about using thalidomide and its analogs (lenalidomide) in first-line treatment of multiple myeloma. Both thalidomide and its analogs, lenalidomide are effective in the treatment of multiple myeloma with lenalidomide having a better effect than thalidomide. In May 2006, the US FDA approved it for the treatment of multiple myeloma. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Treatment of rheumatism | Foreign scholars have reported that when using thalidomide for treatment of 7 rheumatoid patients who can’t be cured by various kinds of anti-inflammatory drugs and immune inhibition, the symptoms were alleviated in most cases within a few weeks at the dose in 400~600mg/d. All patients have their erythrocyte sedimentation rate and rheumatoid factor titers either be normalized or be decreased, wherein 1 case of rheumatoid nodules disappeared at 12 weeks. Someone have ever combined thalidomide with methotrexate for treating 7 cases of refractory rheumatoid arthritis, wherein in 5 cases of patient who persists in treatment, 4 cases obtained alleviated joints tenderness and reduced joints swelling feeling within 3 to 9 months. Thalidomide to treat rheumatism diseases as follows: 1. Behcet's disease. 2. Systemic lupus erythematosus. 3. Rheumatoid arthritis. 4. Erythema nodosum, Crohn's disease. 5. Scleroderma: at 12 weeks after the start of treatment, it can significantly alleviate the symptoms of gastroesophageal reflux, heal duodenal ulcer and lead to hypopigmentation. 6. Adult Still's disease. 7. Refractory ankylosing spondylitis, multiple myeloma (MM). Clinical treatment of rheumatism should start from small dose at 25--50mg/day per night, gradually increase the amount to 100--200mg/day with the maximum not exceeding 400mg/day. |
| Side effects | Adverse reactions during the treatment using thalidomide include: drowsiness, dizziness, drowsiness, headache, constipation, nausea, vomiting, dry mouth, dry skin, erythema and papules and vesicular transient rash; but they usually are not serious and can disappear after termination of administration. The adverse reactions that should be noted is multiple neuritis with the main symptom being a surface or deep sensory loss and muscle weakness; the occurrence of symptoms is not proportional to the dose and duration; the time when the symptoms began to appear also varies greatly; for the cases without treatment termination, such symptoms are irreversible. In addition, leukopenia, abnormal liver function as well as the well-known teratogenic effects also should be taken care. Other rare side effects include bradycardia, edema, abnormal blood clotting, kidney failure, pneumonia, paresthesia, and hypothyroidism. |
| Chemical Properties | White powder. |
| Uses | It is used as sedative and has certain efficacy in treating various types of leprosy reactions such as fever, erythema nodosum, neuralgia, joint pain, and swollen lymph nodes but has no treatment effect on leprosy. |
| Chemical Properties | White Powder |
| Uses | antiinfective (topical) |
| Uses | Inhibits FGF-induced angiogenesis. Inhibits replication of human immunodeficiency virus type 1. Teratogenic sedative. There is now a growing clinical interest in Thalidomide, and it is introduced as an immunomodulatory agent used primarily in combination with dexamethasone to treat multiple myeloma. |
| Definition | ChEBI: A dicarboximide that is isoindole-1,3(2H)-dione in which the hydrogen attached to the nitrogen is substituted by a 2,6-dioxopiperidin-3-yl group. |
| Uses | Thalidomide was prescribed as an anti-nausea agent to help pregnant women with morning sickness in the late 1950s. It was found to be a potent teratogen, causing many different forms of birth defects and was withdrawn from the market. Thalidomide and synthetic analogs have recently been proven effective in treating inflammation associated with diseases such as leprosy, arthritis and Crohn’s disease, and in cancers such as multiple myeloma. The direct target for the teratogenicity of thalidomide was not discovered until 2010, when it was found that it interacts directly with the protein cereblon (CRBN; IC50 = 8.5 nM), a ubiquitously-expressed E3 ligase. Binding of thalidomide analogs to CRBN-DNA damage binding protein-1 complexes account for the immunomodulatory and antiproliferative effects of these compounds.[Cayman Chemical] |
| General Description | Needles or white powder. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | Organic amides/imides, such as Thalidomide, react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). |
| Fire Hazard | Flash point data for Thalidomide are not available; however, Thalidomide is probably combustible. |
| Biological Activity | Teratogen, sedative-hypnotic with inherent anti-inflammatory properties. A selective inhibitor of tumor necrosis factor α (TNF- α ) synthesis. |
| Safety Profile | Poison by ingestion. Moderately toxic by skin contact and intraperitoneal routes. Human teratogenic effects by ingestion: developmental abnormalities of the musculoskeletal and cardiovascular systems. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic and teratogenic data. Human mutation data reported. It was commonly used as a prescription drug in Europe in the late 1950s and early 1960s. Its use was dscontinued because it was lscovered to cause serious congenital abnormalities in the fetus, notably amelia and phocomelia - (absence or deformity of the limbs, including hands and feet) when taken by a woman during early pregnancy. When heated to decomposition it emits toxic fumes of NOx. Used as a sedative and hypnotic. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;


