1/2
Thalidomide NEW
- Min. Order1kg
- Purity99%
- Cas No50-35-1
- Supply Abilityg-kg-tons, free sample is available
- Update time2025-09-25
| Product Name | Thalidomide |
| CAS No | 50-35-1 |
| EC-No | |
| Min. Order | 1kg |
| Purity | 99% |
| Supply Ability | g-kg-tons, free sample is available |
| Release date | 2025/09/25 |
1. Materials information
Names
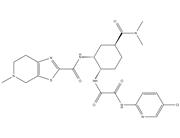
| Name | 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione |
|---|---|
| Synonym | More Synonyms |
Thalidomide Biological Activity
| Description | Thalidomide is initially promoted as a sedative, inhibits ereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM, and has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties. |
|---|---|
| Related Catalog | Signaling Pathways >> Metabolic Enzyme/Protease >> E1/E2/E3 Enzyme Research Areas >> Inflammation/Immunology Research Areas >> Cancer |
| Target | Kd: ∼250 nM (CRL4CRBN)[1] |
| In Vitro | Thalidomide is initially promoted as a sedative, has immunomodulatory, anti-inflammatory and anti-angiogenic cancer properties, and targets ereblon (CRBN), a part of the cullin-4 E3 ubiquitin ligase complex CUL4-RBX1-DDB1, with a Kd of ∼250 nM[1]. Thalidomide (50 μg/mL) potentiates the anti-tumor activity of icotinib against the proliferation of both PC9 and A549 cells, and this effect is correlated with apoptosis and cell migration. In addition, Thalidomide and icotinib inhibits the EGFR and VEGF-R2 pathways in PC9 cells[3]. |
| In Vivo | Thalidomide (100 mg/kg, p.o.) inhibits the collagen deposition, down-regulates the mRNA expression level of α-SMA and collagen I, and significantly reduces the pro-inflammatory cytokines in RILF mice. Thalidomide alleviates RILF via suppression of ROS and down-regulation of TGF-β/Smad pathway dependent on Nrf2 status[2]. Thalidomide (200 mg/kg, p.o.) combined with icotinib shows synergistic anti-tumor effects in nude mice bearing PC9 cells, suppressing tumor growth and promoting tumor death[3]. |
| Cell Assay | THP-1 cells, A549 cells and KYSE30 cells are cultured in RPMI-1640 Medium supplemented with 10% fetal bovine serum and maintained at 37 °C in an atmosphere of 5% CO2 and 95% room air. THP-1 cells is irradiated with a single dose of 4 Gy 6-MV X-ray and treated with or without Thalidomide (0.2 μmol/mL)-containing medium for 48 h after radiation. The concentration of Thalidomide is selected based on the preliminary results[2]. |
| Animal Admin | Mice[2] A total of 24 WT C57BL/6 mice are randomly divided into 4 groups for the experiments (n = 6 in each group): a control group, an irradiated group, a group irradiated along with Thalidomide, and a Thalidomide only group. Based on the preliminary results, 100 mg/kg Thalidomide is used in the experiment. Thalidomide is dissolved in DMSO vehicle. The treatment group receives the indicated dose of Thalidomide in 200 μL by gavage every other day beginning on day 1 for six treatments. The control mice receives 200 μL 0.1% DMSO contained-saline only. The lungs are harvested at 12 weeks after irradiation for the analysis. A total of 20 Nrf2-/- mice are randomly divided into 4 groups for the experiments (n = 5 in each group). The experiment procedures of Nrf2-/- mice are the same as WT C57BL/6 mice. In addition, a total of 30 WT C57BL/6 mice are randomly divided into 5 groups for the subsequent experiments (n = 6 in each group): a control group, an irradiated group, a group irradiated along with CDDO-Me and Thalidomide, a group irradiated along with CDDO-Me, and a group irradiated along with Thalidomide. 600 ng and 100 mg/kg are selected as the dose of CDDO-Me and Thalidomide for the experiment, respectively. The treatment group receives the indicated dose of CDDO-Me or Thalidomide in 200 μL by gavage every other day beginning on day 1 for six times. For the combined group of CDDO-Me and Thalidomide, CDDO-Me is delivered in 200 μL by gavage every other day beginning on day 1 for six treatments. Thalidomide is delivered in 200 μL by gavage every other day beginning on day 2 for six treatments[2]. |
| References | [1]. Fischer ES, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014 Aug 7;512(7512):49-53. [2]. Bian C, et al. Thalidomide (THD) alleviates radiation induced lung fibrosis (RILF) via down-regulation of TGF-β/Smad3 signaling pathway in an Nrf2-dependent manner. Free Radic Biol Med. 2018 Dec;129:446-453. [3]. Sun X, et al. Synergistic Inhibition of Thalidomide and Icotinib on Human Non-Small Cell Lung Carcinomas Through ERK and AKT Signaling. Med Sci Monit. 2018 May 15;24:3193-3203. |
Chemical & Physical Properties
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 509.7±43.0 °C at 760 mmHg |
| Melting Point | 269-271°C |
| Molecular Formula | C13H10N2O4 |
| Molecular Weight | 258.229 |
| Flash Point | 262.1±28.2 °C |
| Exact Mass | 258.064056 |
| PSA | 83.55000 |
| LogP | 0.54 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | Store at RT |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.6 mg/mL | <0.1 g/100 mL at 22 ºC |
MSDS
Thalidomide MSDS(Chinese) |
Toxicological Information
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
Safety Information
| Symbol |   GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360D |
| Precautionary Statements | P201-P280-P301 + P312 + P330-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R46;R61;R21;R25;R62 |
| Safety Phrases | S53-S22-S26-S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | TI4375000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2925190090 |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: mia@fdachem.com
Mob: 86 18336764634
WhatsApp/Skype/Wechat/LINE: 86 18336764634
Company Profile Introduction
Henan Fengda Chemical Co., Ltd. a research customization and supplier specializing in PPb grade ultra-high purity electronic chemicals, deeply rooted in the semiconductor and new display fields, committed to providing the most core and purest chemical components for photoresist and high-end display materials. We provide key monomers for KrF/ArF photoresist, photoacid generators (PAGs), photoacids, and etching solution raw materials, providing cornerstone materials for advanced process photoresist formulations; We take ICP-MS full spectrum metal impurity analysis as the quality cornerstone and provide ICP-MS metal impurity COA reports to meet the strict industry requirements for PPb grade electronic chemical materials. The COA report contains important data such as NA, Mg, K, Ca, Al, Cr, Fe, Ni, Cu, Zn, Pb, Ag, etc. To provide customers with pure and reliable source assurance for cutting-edge research and stable production.


 China
China