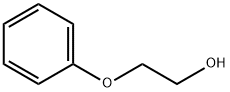
| Chemical Properties |
clear colorless liquid |
| Uses |
Antimicrobial preservative; also used topically in treatment of bacterial infections. |
| General Description |
Colorless liquid with a pleasant odor. Density 1.02 g / cm3. An irritant. |
| Air & Water Reactions |
Oxidizes in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. Water soluble. |
| Reactivity Profile |
2-Phenoxyethanol may react violently with strong oxidizing agents. May generate flammable and/or toxic gases with alkali metals, nitrides, and other strong reducing agents. May initiate the polymerization of isocyanates and epoxides. |
| Health Hazard |
May cause moderate eye irritation and moderate corneal injury. Excessive exposure may cause skin irritation and hemolysis. |
| Fire Hazard |
2-Phenoxyethanol is combustible. |
| Contact allergens |
Phenoxyethanol is an aromatic ether-alcohol used mainly as a preservative, mostly with methyldibromoglutaronitrile (in Euxyl? K 400) or with parabens. Sensitization to this molecule is very rare. |
| Safety Profile |
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. Mutation data reported. Some glycol ethers have dangerous human reproductive effects. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. To fight fEe, use CO2, dry chemical. Used as a solvent for ester-type resins. See also GLYCOL ETHERS. |

 China
China