1/1
Albendazole
$ 1.00
/1KG
- Min. Order1G
- Purity98%
- Cas No54965-21-8
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Albendazole |
| CAS No | 54965-21-8 |
| EC-No | |
| Min. Order | 1G |
| Purity | 98% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
AD68
| Albendazole Basic information |
| Product description Solubility Pharmacodynamics Usage and dosage Side effects Chemical Properties Uses Production method |
| Product Name: | Albendazole |
| Synonyms: | [5-(PROPYLTHIO)-1H-BENZIMIDAZOL-2-YL]CARBAMIC ACID, METHYL ESTER;[5-(PROPYLTHIO)BENZIMIDAZOL-2-YL]CARBAMIC ACID METHYL ESTER;(5-PROPYLSULFANYL-1H-BENZOIMIDAZOL-2-YL)-CARBAMIC ACID METHYL ESTER;ALBAZINE;ALBEN;ALBENDAZOLE;ALBENZA;AKOS NCG1-0064 |
| CAS: | 54965-21-8 |
| MF: | C12H15N3O2S |
| MW: | 265.33 |
| EINECS: | 259-414-7 |
| Product Categories: | Pharmaceutical;Active Pharmaceutical Ingredients;API;Intermediates & Fine Chemicals;Pharmaceuticals;Veterinaries;Miscellaneous Compounds;Aromatics;Heterocycles;Sulfur & Selenium Compounds;Animal Pharmaceuticals;Pharmaceutical intermediate;OVRETTE;Vermifuge |
| Mol File: | 54965-21-8.mol |
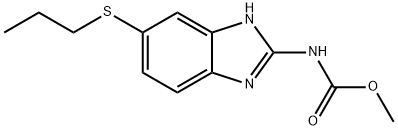 |
|
| Albendazole Chemical Properties |
| Melting point | 208-210 °C |
| density | 1.2561 (rough estimate) |
| refractive index | 1.6740 (estimate) |
| storage temp. | 0-6°C |
| Merck | 14,210 |
| InChIKey | HXHWSAZORRCQMX-UHFFFAOYSA-N |
| CAS DataBase Reference | 54965-21-8(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | T,Xn |
| Risk Statements | 61-36/37/38-48/22-63-33 |
| Safety Statements | 53-45-37/39-26-36/37-24/25 |
| WGK Germany | 2 |
| RTECS | FD1100000 |
| HS Code | 29332990 |
| Hazardous Substances Data | 54965-21-8(Hazardous Substances Data) |
| Albendazole Usage And Synthesis |
| Product description | Albendazole is studied and developed by the company SmithKline Beecham (GSK's predecessor). It is a kind of broad spectrum thiabendazole class anthelmintics which can be applied by both human and animal. It can inhibit the parasite growth and reproduction by inhibiting the intestinal and absorbing the intracellular protein, resulting in the failure of parasite in sugar uptake which is indispensable for their survival, thus causing the endogenous glycogen depletion of insects and further inhibiting fumarate reductase system, preventing the generation of adenosine triphosphate, causing that the parasites are not be able to further survive and reproduce, and ultimately die due to energy depletion. Albendazole has capability of completely killing the eggs of whipworm and hookworm as well as partially killing Ascaris’ eggs; it can also get rid of various kinds of nematodes parasitizing inside animal bodies, and has effects on either getting rid of or directly killing tapeworms and cysticerci. It is thus useful in the treatment of hydatid and the nervous system (cysticercosis) caused by infection of pork worm, and also in the treatment of hookworm, roundworm, pinworm, nematode trichinella, tapeworm, whipworm and stercoralis nematode. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Solubility | It is slightly soluble in acetone or chloroform, but insoluble in water; it is also slightly soluble in hot diluted hydrochloric acid, and soluble in methanol, ethanol, and acetic acid. |
| Pharmacodynamics | Albendazole is a kind of benzimidazole derivatives. It is rapidly metabolized in vivo into the sulfoxide, sulfone and 2-polyamine sulfone alcohol. It can selectively and irreversibly suppress the glucose uptake of intestinal nematodes, thus resulting in endogenous glycogen depletion of the worm; at the same time, it also inhibit the activity of fumarate reductase, and thus preventing the generation of adenosine triphosphate, finally causing death of the parasites. Similar as mebendazole, through causing the denaturation of cytoplasmic microtubules of intestinal parasites and binding to the tubulin, it causes clogging of intracellular transport, causing the accumulation of Golgi endocrine particles; cytoplasm is further gradually dissolved, causing the final death of the parasites. This product can completely kill hookworm eggs, pinworm eggs, spin wool eggs, tapeworm eggs and cysticercosis whip eggs and partially kill Ascaris’ eggs. |
| Usage and dosage | Roundworm disease and pinworm disease: take 400mg daily per time. Hookworm disease, whipworm disease, stercoralis disease: take 400mg each time, 2 times a day, continue for 3 days. Trichinella spirallis disease, take 600mg or 800mg daily in 2 times; a course of treatment is one week. Neurocysticercosis take daily 18mg/kg in 2 times of oral administration; 10 days is a course of treatment; you can also extend the course to one month according to the specific disease situation. Hydatid disease: take 20mg/kg daily in 2 times of oral administration with the course being 1 month; it usually needs multiple times of treatment. |
| Side effects | Adverse reactions include nausea, dizziness, insomnia, dry mouth, fatigue, chills, upset stomach, mild abdominal pain, loss of appetite, etc., they mainly occur in 2-3 days after the administration of the drug; in mild cases, the symptoms can disappear within a few hours with dizziness, fatigue being able to sustaining 2-three days; the incidence is 6-14.9%. Upon treatment of ascariasis, vomiting roundworm occurred in about 2% of cases. When treating cysticercosis especially neurocysticercosis, the reverse reactions are mainly due to the release of heterogonous proteins by the dead cysticercus; it mostly occurs at 2 to 7 days after the medication with symptoms including headache (53.7%), fever (22.7%), rash, muscle aches, vision disorder (4.3%), seizures (13.3%), etc., we shall take appropriate measures (application of glucocorticoid, reducing intracranial pressure, epilepsy treatment, etc.); there has been reports of the dead cases caused by brain herniation during the treatment. Treatment of cysticercosis and echinococcosis which demands a relative large dose and long course of treatment may result in elevated expression of alanine aminotransferase. This will gradually return to normal condition after withdrawal of the drug. |
| Chemical Properties | It is white to light yellow crystalline powder and is odorless. Its melting point is 207-211°C (decomposition). It is slightly soluble in organic solvents but insoluble in water. Rats by oral administration: LD50: 2.4g/kg; sheep by oral administration: LD50:100mg/kg. |
| Uses | 1. It can be used as anthelmintic which is effective in treating both animal gastrointestinal nematodes as well as liver trematode. It can also be supplemented into the feed. 2. It is a kind of efficient broad-spectrum anthelmintic. It is of both broad anti-worm spectrum and highest potency as a kind of benzimidazole derivative drug. It is appropriate for getting rid of roundworm, pinworm, hookworm, and whipworm and for treatment of various types of cysticercosis; it can also be used for getting rid of worm in animal. 3. It can be used to control cabbage downy mildew, rice blast, tobacco powdery mildew, and anthracnose, etc. 4. It is a broad-spectrum anthelmintic. It can be used to get rid of roundworm, pinworm, tapeworm, and whipworm. 5. The product is a high-efficient, broad-spectrum anthelmintic drugs; it has significant efficacy in treating fasciola, tapeworm, and lung & gastrointestinal nematodes. It is one the most potent drugs in benzimidazole derivatives and is listed as the primary choice of drugs in prevention and treatment of livestock and poultry parasitic disease. The product is effective in treating cattle, goat fasciola adults and larvae as well as fasciola gigantic with the worm reduction rate being as high as 90-100%. In recent years, it was found that the product has a strong effect on treating cysticercus bovis. After the treatment, cysticercus number was significantly reduced and the lesions also disappeared. In addition, the product can also be used for preventing parasitic infections, and thus having a good efficacy in promoting the growth of sheep as well as increasing the wool production output. 6. It is a kind of high-efficient and broad-spectrum anthelmintic. It can block the parasites’ absorption on various kinds of nutrition and glucose, resulting in parasite glycogen depletion, and finally causing the failure of the survival and reproduction of the parasite. It can be used to treat single or mixed infection caused by roundworm, pinworm, whipworm, and hookworm. |
| Production method | 1. Take 2-acetamido-4-chloro-nitrobenzene as the starting material, have it react with propyl mercaptan to obtain 2-amino-4-propylthio nitrobenzene which was further converted to 2-amino-4-propylthio aniline undergoing the palladium-charcoal reduction. It is further cyclized with cyanamide methyl formate to obtain albendazole. 2. o-nitroaniline was taken as the starting material; it was first reacted with the sodium thiocyanate to obtain 2-nitro-4-thiocyano aniline and further reacted with bromopropane to generate 2-nitro-4-propylthio aniline which is reduced into 2-amino-propylthio aniline by sodium sulfide; without separation, directly have it be cyclized with cyanamide methyl formate to generate albendazole with the yield being 60%. 3. Take carbendazim as the raw material; have it reacted with sodium sulfocyanide to obtain cyano-benzimidazol-2-carbamate with the yield being 90%; further have reduction reaction, and have it subjected directly to condensation reaction with bromopropane without separation with the yield being 94%. Alternatively, we can also have carbendazim reacted with cyanuric acid to generate 5-sulfonyl chloride-benzimidazol-2-methyl carbamate with a yield of 93%, followed by reduction into 2-mercapto-2-benzimidazole-2-methyl carbamate by iron powder (or zinc powder), and further reaction with bromine propane to obtain the finished product. 4. Sodium sulfide was dissolved in 95% ethanol; add 2-nitro-4-thiocyanate aniline at 50 °C. Stir at 60 °C and cool to 40 °C, pour into bromopropane at once; perform the reaction at 40 °C first and then change to 60 °C for further reaction; add sodium sulfide and have reaction at 80 °C. Distill off the ethanol under reduced pressure, and further add water; Cool and extract with chloroform; the extract was further washed with water; dry; add S-methyl-N, N-bis (methoxycarbonyl) isothiourea and acetic acid for reflux. Distill off the chloroform, add methanol, cool, filtrate, wash with methanol and dry to obtain the crude albendazole product. 5. Under the stirring and ice-cooling, add carbendazim slowly into chlorosulfonic acid carbendazim while keeping the internal temperature at 15~20 °C. After the completion of addition, stir at 40 °C. At below 0 °C, slowly add drop wise of the reaction mixture into the 95% ethanol. The precipitate was collected by filtration and washed with a small amount of ethanol, and dried to obtain the chloro-sulfonated product with a yield of 90.8%. The chloro-sulfonated product was further added into the aqueous solution containing methanoic acid, water and 40% hydrobromic acid together with aluminum metal powder. Stir at 35~40°C first, and then have reaction at 60~65 °C. After cooling and filtration, the filtrate was adjusted with 40% sodium hydroxide to pH value of 3. The precipitate was collected by filtration, washed, and dried to obtain mercapto compound in a yield of 82.5%. The mercapto compound is further added to an aqueous solution of sodium hydroxide, and bleached by activated carbon. Add tetrabutylammonium bromide, and then the methanol solution of bromopropane for reaction at 35~40 °C; cool, filter the precipitate, wash to neutrality and dry to obtain the white crystals of albendazole with the yield being 87.5% and m.p. being 207~209 °C. In the presence of glacier acetic acid, carbendazim is reacted with sodium thiocyanate; after the introduction of sulfur cyano group, then further use sodium sulfide for reduction, followed by reaction with bromopropane which can also obtain the albendazole. Albendazole can be refined using the following method: The crude albendazole product (calculated by dry product amount) was stirred together with 85% industrial methanoic cid (1: 3.5) and rose to 60 °C. The complete dissolution of the crude product was followed sequentially by adding sodium dithionate, activated carbon and EDTA. Continue stirring for warming up to 80 °C, keep the temperature, further subject to suction filtration with the filtrate being poured into deionized water while neutralizing with 18% to 20% aqueous ammonia upon stirring until to a Ph value of 6.5; apply suction filtration again and dry to obtain the white product with mp being 206~212 °C and the refinement yield being 93%~94%. |
| Chemical Properties | Colourless Crystalline Solid |
| Uses | progestogen |
| Uses | An anthelmintic |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;