In 2002, establish in Jun with 8 employees, and the first commercial product: Calcium folinate (Leucovorin calcium).
Thanks to our strong R&D, Jinkang build it’s own strong products step by step, such as Calcium folinate (2MT/p.a. capacity), Mesna (5MT/p.a. capacity), Irinotecan (200kg/p.a. capacity), etc.
In 2006, launch Gemcitabine in 2006 and become the leader in China since 2007. New dedicated workshop for Gemcitabine began to operation in 2008. The capacity of T6 became 40MT per annual.
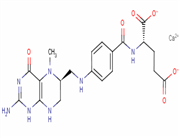
In 2009, launch Pemetrexed in 2009. The capacity of Pem-7 was 500kg in 2009, will become 1MT in 2010.
In Apr. 2010, get the first U.S. patent authorized.
In Aug. 2010, purchase a land about 67 thousand square meters in the city pharmaceutical industrial park to build a new API factory completely complying with cGMP.
In Oct. 2010, get invested from Jiangsu Venture Capital co., ltd.
At the end of Nov., 2010, Jinkang is a company with twelve million USD turnover, 125 qualified and well-trained employees, 2 multi-purpose workshops, 2 dedicated workshops, 1 well-equipped in-site Q/C lab, 2 U.S. patents authorized and 2 other applied, 3 Chinese patents authorized and 2 other applied.

 China
China