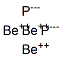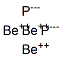Beryllium phosphide has the formula of Be3P2 and
the molecular weight of 88.9841 g/mol. It can be
prepared by reaction of the elements, but not from
aqueous solution:
12Be + 2P4 ? 4Be3P2
Red phosphorus melts at 317.3 C and the boiling
point is 553°C so that addition of Be metal serves to
form the phosphide. By adding Be to P4 at about
350°C, but less than the phosphorus sublimation
temperature of 415°C in an inert atmosphere, the melt
readily forms the phosphide. A better way is to sublime
the P4 at 450°C in an inert gas stream and react it with Be
metal at 750°C. Be3P2 has the CAS number 58127-61-0. It
is a brown powder having a slight odor of garlic. Once
formed, Be3P2 is very stable. However, it reacts slowly
with moisture to form phosphine, PH3 (which is toxic
and flammable), and must be stored under dry
conditions.
Beryllium phosphide is a semiconductor and has
been studied by XRD methods using powder or a crystal,
and by neutron diffraction from powder. example, is 160% wider. It is for this reason that beryllium
phosphide films or composites have found little
usage in the industry.