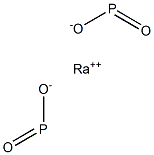
Radium hypophosphite would have the molecular
formula of Ra(H2PO2)2 and the molecular weight of
740.0237 g/mol. It has no CAS number. It has never
been prepared but could be formed by reacting the oxide
with hypophosphorous acid:
RaO + 2H3PO2 ? Ra(H2PO2)2 +H2O
It would expected to be water soluble and would
have strong reducing properties. In view of the intense
radioactivity of 226Ra, it is unlikely that this compound
will ever be synthesized.