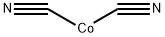The anhydrous form is a deep-blue powder; hygroscopic; density 1.872 g/cm3; melts at 280°C; insoluble in water. The dihydrate is pink to reddish brown powder or needles; insoluble in water and acids; soluble in sodium or potassium cyanide solutions, ammonium hydroxide, and hydrochloric acid.
The compound has limited commercial applications. It is used as a catalyst and in the preparation of cyanide complexes.
The trihydrate salt is obtained as a reddish brown precipitate by adding potasium cyanide to a cobalt salt solution:
CoCl2 + KCN + 3H2O → Co(CN)2?3H2O + 2KCl
This on dehydration yields anhydrous Co(CN)2. The Co(CN)2?3H2O precipitate formed above redissolves when excess KCN is added, forming a red solution of potassium cobalt(II) cyanide, K4Co(CN)6. Stoichiomtric amount of KCN should, therefore, be used in the preparation of cobalt(II) cyanide.
The compound is highly toxic by ingestion and possibly through other routes of exposure.