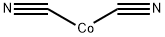Physical properties
The anhydrous form is a deep-blue powder; hygroscopic; density 1.872 g/cm
3; melts at 280°C; insoluble in water. The dihydrate is pink to reddish brown powder or needles; insoluble in water and acids; soluble in sodium or potassium cyanide solutions, ammonium hydroxide, and hydrochloric acid.
Preparation
The trihydrate salt is obtained as a reddish brown precipitate by adding potasium cyanide to a cobalt salt solution:
CoCl
2 + KCN + 3H
2O → Co(CN)
2?3H
2O + 2KCl
This on dehydration yields anhydrous Co(CN)
2. The Co(CN)
2?3H
2O precipitate formed above redissolves when excess KCN is added, forming a red solution of potassium cobalt(II) cyanide, K
4Co(CN)
6. Stoichiomtric amount of KCN should, therefore, be used in the preparation of cobalt(II) cyanide.