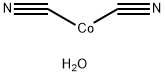
Structure and conformation
A light brown precipitate of Co(CN)2.2 or 2·5H2O can be obtained
from cobalt(II) chloride and potassium cyanide solutions. This can be dehydrated at 250°in a stream of nitrogen to the blue anhydrous cyanide. Both the hydrated and the anhydrous compounds have magnetic moments (3.27 and 312 B.M. respectively) which are much lower than is expected for three unpaired electrons. The crystal structures of these compounds have been interpreted as being that of soluble Prussian blue with one cobalt atom being low spin (one unpaired electron) surrounded by six carbon atoms while the other is high spin (three unpaired electrons) being surrounded by six nitrogen atoms as nearest neighbours.