The reaction is carried out in a round-bottom flask equipped
with a separatory funnel and gas outlet tube connected to a P2O5-
glass wool drying tube. Pure Br2 (100 g.) is allowed to flow slowly
into a suspension of 20 g. of pure powdered Se in 50 ml. of dry CS2.
Finely crystalline yellow SeBr4 separates. When the addition is
complete, a gas inlet tube is substituted for the separatory funnel
and the CS2 and excess Br2 are driven off with a dry air stream.
The residual SeBr4 is rapidly transferred into a tightly sealed
vessel.
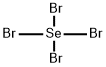
Se + 2Br2 = SeBr4