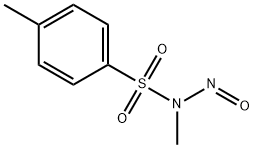
A 10% to 40% aqueous solution of potassium hydroxide (20 mL) was added to a solvent mixture of tetrahydrofuran and methanol containing tert-butyl-2-oxo-7-azaspiro[3.5]nonane-7-carboxylic acid (8.2 g). The solvent ratio was 10:1. In another vessel, N-methyl-N-nitroso-p-toluenesulfonamide (19.6 g) was dissolved in 300 mL of water and added slowly and dropwise at 0 °C to the above reaction solution. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the system was cooled to room temperature and the reaction was quenched by the addition of acetic acid. Subsequently, the reaction mixture was rotary evaporated to dryness and the residue was dissolved in ethyl acetate (100 mL) and washed sequentially with water (100 mL) and saturated brine (100 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification by column chromatography afforded ethyl tert-butyl-2-oxo-8-azaspiro[4.5]decane-8-carboxylate (6.6 g, yield: 72%).
![tert-butyl 2-oxo-8-azaspiro[4.5]decane-8-carboxylate Structure](/CAS/GIF/1250994-14-9.gif)
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)