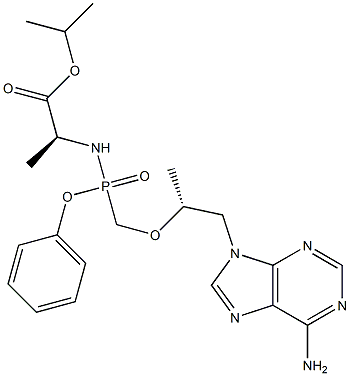
Tenofovir Alafenamide (GS-7340) is a prodrug of tenofovir, which is a reverse transcriptase inhibitor, used to treat HIV and Hepatitis B.-Reverse Transcriptase inhibitor. It was developed by Gilead Sciences. Compared to tenofovir disoproxil fumarate, tenofovir alafenamide has a greater antiviral activity and better distribution into lymphoid tissues.
Tenofovir alafenamide fumarate is an oral phosphonoamidate
prodrug of the reverse transcriptase inhibitor tenofovir. It was
approved by the USFDA for the treatment of chronic hepatitis
B virus infection with compensated liver disease. Tenofovir
alafenamide fumarate was discovered and developed by Gilead
as a potentially safer form of the previously approved tenofovir
disoproxil fumarate (Viread).
Tenofovir Alafenamide, also known as Tenofovir Impurity 51, is a prodrug of Tenofovir (T018500), which is a reverse transcriptase inhibitor used to treat HIV and Hepatitis B.
ChEBI: An L-alanine derivative that is isopropyl L-alaninate in which one of the amino hydrogens is replaced by an (S)-({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)(
henoxy)phosphoryl group. A prodrug for tenofovir, it is used (as the fumarate salt) in combination therapy for the treatment of HIV-1 infection.
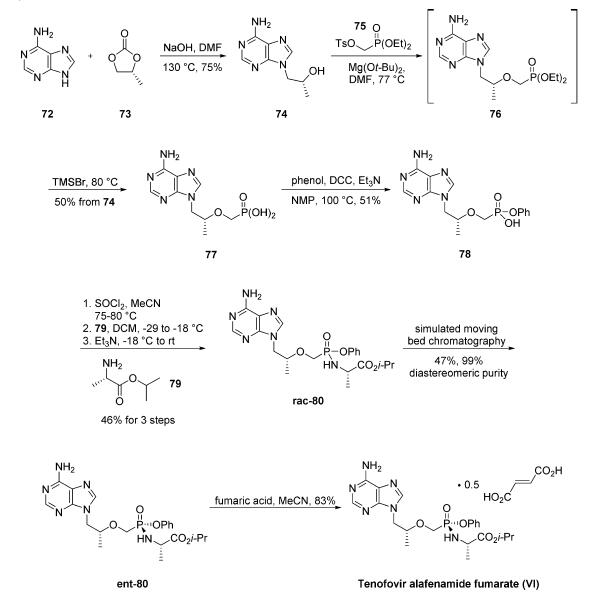
A multikilogram synthesis of tenofovir alafenamide fumarate
was described in a Gilead patent. Additional process
improvements on specific steps of the Gilead process have
been reported on 100 g scale, and these will be noted
throughout the description of the synthesis. The synthesis was
initiated with the alkylation of adenine (72) with (R)-propylene
carbonate (73) to give hydroxypropyl adenine 74 in 75% yield. It should be noted that sodium hydroxide can be
replaced by potassium bases with increased yields on 100 g
scale.27 Alkylation of 74 with diethyl p-toluenesulfonyloxymethylphosphonate
(75) gave intermediate 76, which was not
isolated. Hydrolysis of the phosphonate esters with trimethylsilyl
bromide followed by recrystallization from water gave
phosphonic acid 77 in 50% yield. Interestingly, replacing
Mg(Ot-Bu)2 with PhMgCl/t-BuOH led to improved yields for
the alkylation step (74 ?ú 76) on a 100 g scale. Additionally,
the authors note that conditions for hydrolyzing the
phosphonate ester can be modified using HCl or HBr for
improved yields on smaller scale. Dicyclohexylcarbodiimide
(DCC) coupling of 77 with phenol produced phosphonate 78
in 51% yield. This step was also reported to proceed in higher
yield on smaller scale by changing the solvent to cyclopentylmethyl
ether. Monophosphonate ester 78 was treated
with thionyl chloride followed by L-alanine isopropyl ester (79)
and triethylamine to give tenofovir alafenamide rac-80 as a
mixture of phosphonate diastereomers in 47% yield. The
diastereomers were separated using simulated moving bed
chromatography to give the desired diastereomer ent-80 in
47% yield and 99% diastereomeric purity. The diastereomers
could also be separated using a crystallization-induced dynamic
resolution of rac-80. Tenofovir alafenamide fumarate (VI)
was prepared from ent-80 and fumaric acid in 83% yield.