It is a yellowish liquid, bp 130 ?C/0.1 mmHg, vp 9.3 mPa
(20 ?C). It ismiscible with water and most organic solvents
except kerosene. Log Kow = ?0.5. Dicrotophos is rather
stable to heat and slowly hydrolyzed in acidic media and
more rapidly in alkaline media; DT50 (20 ?C) at pH 5, 7,
and 9 are 88, 72, and 28 d, respectively.
Dicrotophos is an amber liquid with a mild
ester odor.
Dicrotophos is used to control sucking, chewing and boring
insects and mites in a wide range of crops. It is also used as an animal
ectoparasiticide.
Contact and systemic insecticide and acaricide used to control pests on rice, cotton, maize, soybeans, coffee, citrus and potatoes.
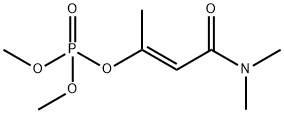
Dicrotophos is synthesized by the Perkow reaction
from trimethyl phosphite and N,N-dimethyl-α-
chloroacetoacetamide, consisting mainly of the (E)-form.
ChEBI: Dicrotophos is a dialkyl phosphate and an organophosphate insecticide. It has a role as an EC 3.1.1.7 (acetylcholinesterase) inhibitor, an acaricide, an avicide and an agrochemical.
A yellow to brown liquid with a mild ester odor. Used to control sucking, boring, and chewing pests on rice, cotton, coffee, apples, and other crops. Effective on ornamentals, trees, and shrubs for aphids, leaf hoppers, and scale insects.
Organophosphates, such as DICROTOPHOS, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. DICROTOPHOS is incompatible with the following: Metals [Note: Corrosive to cast iron, mild steel, brass & stainless steel.] .
DICROTOPHOS is extremely toxic. Probable human oral lethal dose is 5 to 50 mg/kg, 7 drops to one teaspoonful for a 70 kg (150 lb.) person. Closely related in toxicity to azodrin.
An extremely toxic organophosphorus pes-ticide; cholinesterase inhibitor. Toxic effectsare similar to those of monocrotophos. Toxicsymptoms include headache, dizziness, muscle spasms, blurred vision, dilation of pupil,nausea, vomiting, diarrhea, abdominal pain,and seizures. Like most other organophosphorus compounds, exposure to this pesticidecan dangerously affect the respiratory system, producing shortness of breath and respiratory depression, which can progress torespiratory paralysis. Dermal exposure mayincrease the heart rate, while oral intake maydecrease the heart rate. This compound canbe hypotensive and psychotic. Ingestion of asmall quantity of the liquid (0.5–2 g) can befatal to adult humans.
LD50 oral (rat): 15 mg/kg
LD50 skin (rat): 42 mg/kg
LC50 inhalation (rat): 0.09 mg/L/4 h (RTEC1985).
(Non-Specific -- Organophosphorus Pesticide, Liquid, n.o.s.) DICROTOPHOS may burn but does not ignite readily. Container may explode in heat of fire. Fire and runoff from fire control water may produce irritating or poisonous gases. Rapidly hydrolyzes in acid or alkali. Keep away from heat and open flame.
Insecticide, Acaricide: EPA restricted Use Pesticide (RUP). Not approved
for use in EU countries. Dicrotophos was introduced in
1956 as a contact systemic pesticide with a wide range of
applications. Today, dicrotophos is currently used mainly
as an insecticide for apples and other fruit crops, and for
cotton pests, mostly in the Mississippi Valley. It is acutely
toxic to birds, especially those that follow their migratory
corridors and feed in the farmlands that have been treated
with this pesticide. Internationally, dicrotophos is used on
rice, coffee and citrus. One of the major degradates of dicrotophos
is monocrotophos. All uses of monocrotophos
have been voluntarily cancelled in the United States due to
its extreme toxicity to humans and wildlife
BIDIRL®; BIDRIN®; BIDRIN-R®[C];
BIDRIN®[C]; C-709®; C-709®; CARBICRIN®;
CARBICRON®; CARBOMICRON®; CIBA 709®;
DIAPADRIN®; DICRON®; DIDRIN®; EKTAFOS®;
EKTOFOS®;
Poison by ingestion,
inhalation, skin contact, subcutaneous,
intravenous, and intraperitoneal routes.
Mutation data reported. Used to control the
coffee borer and certain economically
important pests of cotton. When heated to decomposition it emits very toxic fumes of
NOx and POx. See also ESTERS.
A potential danger to those involved
in the manufacture, formulation and application of this
organophosphate. Used to control the coffee borer and certain economically important pests of cotton
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. Medical observation is recommended; effect maybe delayed.Note to physician: 1,10-Trimethylenebis(4-formylpyridinium bromide) dioxime (a.k.a TMB-4 dibromide and TMV-4) has been used as an antidote for organophosphatepoisoning.
When rats were fed dicrotophos
in their diets at concentrations of 0, 1, 10, or 100 ppm for
2 years, there were no detectable effects at the 1 ppm
concentration . Plasma cholinesterase was inhibited
at 1 ppm . At 10 and 100 ppm, decreased body weights
and reduced cholinesterase (erythrocyte, plasma, and brain
not specified) activities occurred. Dogs given dicrotophos in
their diets at 0, 0.16, 1.6, or 16 ppm for 2 years showed some instances of slightly excessive salivation . At 16 ppm,
both plasma and RBC cholinesterase activity was decreased.
Soil. The dimethylamino group is converted to an N-oxide then to -CH2OH and aldehyde groups which further degrade via demethylation and hydrolysis (Hartley and Kidd, 1987). Dicrotophos is rapidly degraded under aerobic and anaerobic conditions forming N,N-dimethylacetoacetamide and 3-hydroxy-N,N-dimethylbutyramide as the major metabolites. Other metabolites included carbon dioxide and unextractable residues. The half-life of dicrotophos in a Hanford sandy loam soil was 3 days (Lee et al., 1989).Biological. Identified metabolites of dieldrin from solution cultures containing Pseudomonas sp. in soils include aldrin and dihydroxydihydroaldrin. Other unidentified byproducts included a ketone, an aldehyde and an acid (Matsumura et al., 1968; Kearney and Kaufman, 1976). A pure culture of the marine alga, namely Dunaliella sp., degraded dieldrin to photodieldrin and an unknown metabolite at yields of 8.5 and 3.2%, respectively.
Chemical/Physical. Dicrotophos emits toxic fumes of phosphorus and nitrogen oxides when heated to decomposition (Sax and Lewis, 1987; Lewis, 1990).
Dicrotophos is hydrolyzed in sodium hydroxide solutions forming dimethylamine. The hydrolysis half-lives at 38°C and pH values of 1.1 and 9.1 are 100 and 50 days, respectively (Sittig, 1985). Lee et al. (1989) reported that the hydrolysis half-lives of di
The metabolic fate of dicrotophos mirrors very closely that of its
close congener, monocrotophos. Technical dicrotophos contains 82%
of the E-isomer and 6% of the Z-isomer, the balance being various
impurities. Most studies on the metabolic fate of this compound have
used a purified material containing >99% E-isomer. Dicrotophos is a
systemic vinyl phosphate insecticide with a high water solubility and
low log Kow.
The main routes of metabolic degradation are demethylation to des-
O-methyldicrotophos and hydrolysis to dimethyl phosphate and N,N-dimethylacetoacetamide.
In animals and plants, hydroxylation of the
N-methyl group followed by N-demethylation are also important from a
toxicological point of view, since these metabolic steps yield highly
active idubitors of acetylcholinesterase. In the case of dicrotophos,
N-demethylation affords monocrotophos as one of the products of
metabolism.
The main degradation routes are O-demethylation to
des-O-methyldicrotophos and hydrolysis to dimethyl phosphate
and N,N-dimethylacetoacetamide. Oxidative Ndemethylation
also occurs.
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from strong oxidizers, metals, strongbases, and heat. Where possible, automatically pump liquidfrom drums or other storage containers to processcontainers.
UN3018 Organophosphorus pesticides, liquid,
toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Dicrotophos is relatively stable at acidic and neutral pH values but it
is hydrolysed in alkaline solutions (PM). The DT50s at pH 5, 7 and 9
(25 °C) were 117, 72 and 28 days, respectively. The products of
hydrolysis, which were identified by GC of their methyl esters, were
N,N-dimethylacetoacetamide (2), the main product at alkaline pH
values, and des-O-methyldicrotophos (3), which predominated in acid
and neutral solutions (Lee ef al., 1989). Further decomposition of N,N-dimethylacetoacetamide
(2) under alkaline conditions then gave acetone
(4), CO2 and dimethylamine (5), whereas under acidic conditions the
des-O-methyldicrotophos (3) was shown to decompose to inorganic
phosphate (6), monomethyl phosphate (7), methanol (8), acetone (4) and
N,N-dimethylacetoacetamide (2) (Brown et al., 1966). Dicrotophos is not
subject to photodecomposition. Photolysis experiments in which aqueous
solutions of monocrotophos were exposed to simulated sunlight (xenon
arc lamp) in quartz tubes showed no products other than the hydrolysis
products 2 and 3, neither was their rate of formation accelerated. A
soil surface photolysis experiment indicated some small loss of dicrotophos,probably as CO2 due to microbial action. No particular photolytic
degradation products were detected apart from minor amounts of the
hydrolysis products 2 and 3. After 30 days 80% of the dicrotophos was
recovered unchanged. Residual metabolites which were unextractable
from the soil were not generated by photodegradation, since a dark control
experiment produced the same proportion of unextractable material
(Lee et al., 1989) (see Scheme 1).
The acute oral LD50 for
rats is 17–22 mg/kg. Inhalation LC50 (4 h) for rats is
about 0.09 mg/L air. NOEL in a three-generation reproduction
study with rats is 2 mg/kg daily. In mammals,
orally administered dicrotophos is rapidly metabolized,
and 63–71% was excreted in the urine within 48 h.
Attacks some metals: Corrosive to cast
iron, mild steel; brass, and stainless steel l304. Decomposes
after prolonged storage, but is stable when stored in glass
or polyethylene containers with temperatures to 40�C.
Contact with oxidizers may cause the release of phosphorous oxides. Contact with strong reducing agents, such as
hydrides, may cause the formation of flammable and toxic
phosphine gas
Dicrotophos decomposes after
7 days @ 90�C and 31 days @ 75�°C. Hydrolysis is 50%
complete in aqueous solutions @ 38�C after 50 days at pH
9.1 (100 days are required at pH 1.1). Alkaline hydrolysis
(NaOH) yields (CH3)2NH. Incineration is also recommended as a disposal method. In accordance with
40CFR165, follow recommendations for the disposal of
pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting
your local or federal environmental control agency, or by
contacting your regional EPA office