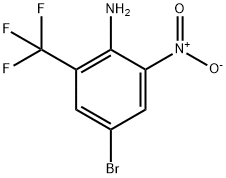
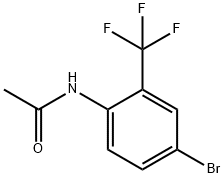
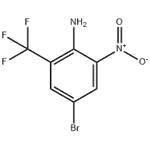
Step B: Synthesis of 4-bromo-2-nitro-6-(trifluoromethyl)aniline
1. Cool sulfuric acid (350 mL) to 5°C in an ice bath.
2. add N-(4-bromo-2-(trifluoromethyl)phenyl)acetamide (100 g, 355 mmol) in one batch, controlling the internal temperature between 5°C and 10°C. 2. add N-(4-bromo-2-(trifluoromethyl)phenyl)acetamide (100 g, 355 mmol) in one batch.
3. after the solid is completely dissolved and the temperature is reduced to 5°C, slowly add 70% nitric acid (43.8 mL) dropwise, making sure the internal temperature does not exceed 10°C.
4. Maintain the reaction temperature between 8°C and 12°C until the reaction is complete.
5. Carefully pour the reaction solution into stirred ice water (2 L, mostly ice).
6. The tan precipitate was collected by filtration and the filter cake was washed with cold water (1 L) and subsequently air dried.
7. The resulting tan powder was placed in a 3 L three-necked flask and methanol (500 mL) was added to make a slurry.
8. 37% concentrated hydrochloric acid (750 mL, 9130 mmol) was added and heated to 100°C using a heating shield. care was taken to control excessive foaming.
9. To reduce foaming, 1,4-dioxane (100 mL) and tetrahydrofuran (200 mL) were added.
10. lower the reaction temperature to 80°C and continue heating overnight for better foam control.
11. Slowly increase temperature to 95°C with stirring for 72 hours.
12. cool to room temperature and extract three times with ethyl acetate.
13. The organic layers were combined, washed with brine, dried over sodium sulfate and concentrated to give 2-amino-5-bromo-3-nitrobenzotrifluoride (101 g, 99%).
1H NMR (400 MHz, DMSO-d6) δ ppm: 8.40 (d, 1H), 7.96 (d, 1H), 7.44 (br.s, 2H).