Acetyl Isoeugenol is used as an alcohol-soluble flavoring agent with spicy, floral, and carnation undertones, blending well with vanilla-type flavors. It lasts longer in perfume than other comparable agents such as dihydroeugenol and methyl diantilis, giving body to the scent. It can be used as an alternative to products such as dianthine, which degrades into isoeugenol – which can cause hives in some individuals from long-term exposure, and has thus been deemed non-IFRA (International Fragrance Association) compliant.
http://www.abchemicalindustries.com/acetyl-iso-eugenol-2394883.html
http://www.basenotes.net/threads/413562-Iso-Eugenol-replacer
http://www.organicaaroma.com/products/synthetic/acetyl-iso-eugenol
https://en.wikipedia.org/wiki/Isoeugenol
http://www.basenotes.net/threads/407876-Vintage-Dianthine-Base-Formula
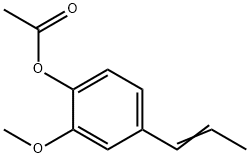
Isoeugenyl acetate has a weak, rose-carnation, somewhat spicy
odor and an initially burning, then sweet taste. Can be prepared
from isoeugenol and acetic acid by esterification.
Isoeugenyl acetate has a weak, rose–carnation, somewhat spicy, clove-like odor and an initially burning, then sweet,
taste.
Has apparently not been reported to occur in nature
Isoeugenyl acetate is a useful building block extracted from the essential oils of Alpinia galanga and Zingiber officinale. Isoeugenyl acetate has antibacterial properties towards B. subtilis, E. coli and S. aureus. Useful in floral perfume compositions, as a sweetener in herbaceous fragrances, and as a fixative in carnation perfumes. May cause discoloration in white soaps. In flavors for berry, fruit and spice compositions. Concentrations from 0.4 to 17 ppm. in finished products. Up to 100 ppm in chewing gums.
From isoeugenol and acetic acid by esterification.
ChEBI: Isoeugenol acetate is a phenylpropanoid that is the acetate ester of trans-isoeugenol. It is a phenylpropanoid, a monomethoxybenzene and a member of phenyl acetates. It is functionally related to a trans-isoeugenol.
Moderately toxic by ingestion. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
The hydrolysis of ester linkages of foreign compounds may be catalysed by many different esterases, which are to be found in all animals and bacteria and which generally have a low degree of substrate specificity(Parke, 1968).