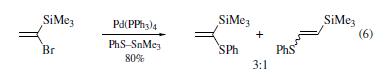
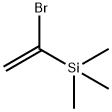
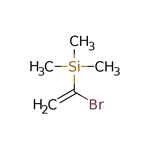
Numerous nucleophiles
react with (1-bromovinyl)trimethylsilane in the presence of
palladium complexes. The bromine has been substituted by
phenylthio,vinyl,and aryl groups. This approach gives reasonable
yields of the desired products. However, the substitution
reactions sometimes lack regiospecificity. For example, a mixture
of regioisomers was obtained in eq 6. Two mechanisms have been
proposed for the formation of the β-substituted product. One involves
an elimination step to give trimethylsilylacetylene as an
intermediate, which then undergoes catalyzed additions with the
nucleophile at either the α- or β-positions.The other mechanism
involves the formation of a pentacoordinated palladium intermediate,
leading to the formation of isomeric products.