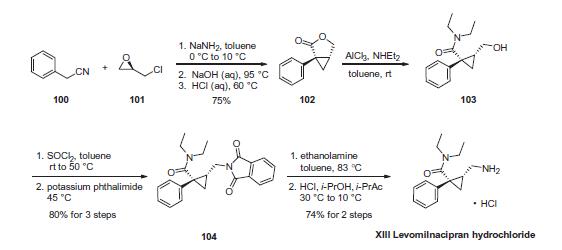
Reaction of phenylacetonitrile (100) and commercially available
(R)-epichlorohydrin (101) with NaNH2 led to chloride displacement
and intramolecular cyclopropanation, yielding lactone 102
after a one-pot nitrile hydrolysis and acid-promoted lactonization (75% yield over 3 steps). Lactone ring-opening with Et2NH¨CAlCl3
complex provided amido-alcohol 103, which was converted to its
phthalimido derivative 104 by sequential treatment with thionyl
chloride and potassium phthalimide in 80% over three steps.
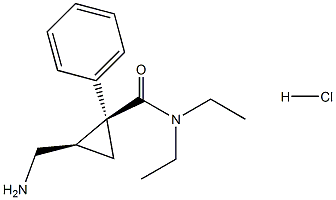
Finally, levomilnacipran hydrochloride (XIII) was obtained in
>95% optical purity after phthalimide cleavage, HCl salt formation,
and crystallization from HCl/i-PrOH/i-PrAc. This sequence represents
a highly efficient route to levomilnacipran, requiring no isolation
of intermediates, resulting in >40% overall yield, and
allowing use of the same solvent solution (toluene) for all steps.