DDD, 1,1-dichloro-2,2-bis-(4-chlorophenyl) ethane, also known as TDE, is a
metabolite of DDT, which found use in agriculture. As with DDT, the p,p -isomer is insecticidal and the o,p -isomer is an impurity.
For determination of protein-bound sulfhydryl groups.
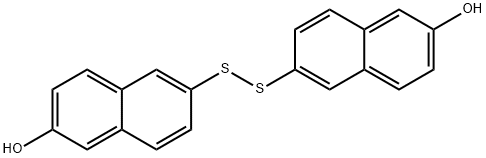
It crystallises as leaflets from AcOH and is slightly soluble in EtOH, and AcOH, but is soluble in *C6H6 and in alkalis to give a yellow solution. [Zincke & Dereser Chem Ber 51 352 1918.] The acetoxy derivative has m 198-200o (from AcOH or dioxane/MeOH), and the diacetyl derivative has m 167-168o (from AcOH). A small amount of impure disulfide can be purified by dissolving it in a small volume of Me2CO and adding a large volume of toluene, filter rapidly and concentrate to one-third of its volume. The hot toluene solution is filtered rapidly from any tarry residue, and crystals separate on cooling. Recrystallisation from hot acetic acid gives crystals with m 220-223o [Barrett & Seligman Science 116 323 1952]. [Beilstein 6 I 481.]
Animal Studies. DDD is less acutely toxic
than DDT (29), with an oral LD50 of 3400 to 4000 mg/kg in
the rat and a dermal LD50 ca. 1200 mg/kg in the rabbit. In a
2-year chronic dietary study in the rat, a LOEL of 100 ppm
(5 mg/kg/day) was quoted for tissue damage, similar to that
produced by DDT.
Human Studies. Most of the studies conducted in
humans have used the o,p -isomer of DDD. This has been
used as a drug, with medical and veterinary uses, for the
treatment of Cushing’s syndrome and adrenal carcinoma,
under the generic name of mitotane. After oral ingestion,
o,p’-DDD binds to lung and adrenal tissue. Its toxicity to the
adrenal appears to be species-dependent, however, causing
gross atrophy in the dog and reducing corticosteroid
production without pathological effects in other mammals,
including humans.