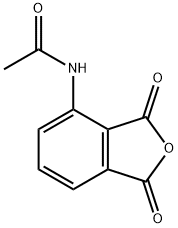
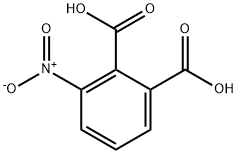
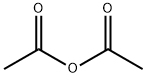
General procedure for the synthesis of N-(1,3-dioxo-1,3-dihydroisobenzofuran-4-yl)acetamide from 3-nitrophthalic acid and acetic anhydride: 3-nitrophthalic acid (100.0 g) and acetic anhydride (1 L) were added to a hydrogenation kettle and heated to 80 °C until the raw materials were completely dissolved. Subsequently, the reaction mixture was cooled to room temperature and 10% Pd/C catalyst (3 g) was added. The atmosphere in the reaction kettle was replaced twice with nitrogen, then hydrogen was introduced to a pressure of 1 MPa, and the reaction was kept at room temperature until hydrogen absorption stopped. Upon completion of the reaction, the hydrogen was vented and the reactor was purged with nitrogen. Next, the reaction mixture was heated to 110-115 °C until the feedstock or intermediate completely disappeared. Thermal filtration was carried out and the filtrate was cooled to 0-5 °C, the product was collected by filtration and washed with glacial acetic acid. A final light yellow crystalline product of 80.6 g was obtained with 83% yield and 99.0% HPLC purity.