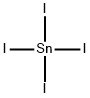
TIN(IV) IODIDE
- Product NameTIN(IV) IODIDE
- CAS7790-47-8
- MFI4Sn
- MW626.33
- EINECS232-208-4
- MOL File7790-47-8.mol
Chemical Properties
| Melting point | 144 °C |
| Boiling point | 364 °C |
| Density | 4.47 g/mL at 25 °C(lit.) |
| refractive index | 2.106 |
| Flash point | 340°C |
| solubility | Soluble in alcohol, benzene, carbon disulfide, chloroform and ether. |
| form | powder |
| color | Red |
| Specific Gravity | 4.473 |
| Water Solubility | Soluble in alcohol, benzene, CS{2}, chloroform, and ether. Decomposes in water |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Sensitive | Moisture Sensitive |
| Merck | 14,8777 |
| Exposure limits | ACGIH: TWA 2 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 2 mg/m3 |
| Major Application | electroplating material synthesis precursor perovskite solar cells |
| InChI | 1S/4HI.Sn/h4*1H;/q;;;;+4/p-4 |
| InChIKey | QPBYLOWPSRZOFX-UHFFFAOYSA-J |
| SMILES | I[Sn](I)(I)I |
Safety Information
| Hazard Codes | C |
| Risk Statements | 20/21/22-34-42/43 |
| Safety Statements | 26-28-36/37/39-45 |
| RIDADR | UN 3260 8/PG 2 |
| WGK Germany | 3 |
| RTECS | XQ3675000 |
| F | 1-8-10-19 |
| TSCA | TSCA listed |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28276000 |
| Storage Class | 8A - Combustible corrosive hazardous materials |
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Inhalation Acute Tox. 4 Oral Eye Dam. 1 Resp. Sens. 1 Skin Corr. 1B Skin Sens. 1 |
| Toxicity | MLD in rats (mg/kg): 200 i.v. (Kolmer) |
