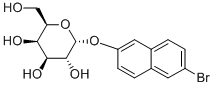
It is prepared from penta-O-acetyl-D-galactoside, 6-bromo-2naphthol and ZnCl2. The resulting tetra-acetate (2g) is hydrolysed by dissolving in 0.3N KOH (100mL) and heating until the solution is clear, then filtering and cooling to give colourless crystals of the -isomer which are collected and recrystallised twice from hot MeOH. The high specific rotation is characteristic of the isomer. The tetraacetate has m 155-156o, [] D 20 +60o (c 1, CHCl3) [Dey & Pridham Biochem J 115 47 1969] [reported m 75-85o, [] D 24 +94o (c 1.3, dioxane), Monis et al. J Histochem Cytochem 11 653 1963]. [Beilstein 17 IV 2972.]