3-n-Butyl-6-methyl-3,4-dihydro-1,2-pyran was obtained and isolated from the
reaction mixture by fractional distillation in vacuum to separate a by-product
formed by cyclodimerisation of methyl vinylketone. The yield of the3-n-butyl-
6-methyl-3,4-dihydro-1,2-pyran was 47% (boiling point 106-107°C.
The saponification of the 3-n-butyl-6-methyl-3,4-dihydro-1,2-pyran was
accomplished by heating for 0.5 h in a mixture with acetic acid. The 1-methyl-
4-n-butyl-1,5-dicarbonyl acid formed not isolated from the reaction mixture
was added to hydroxylamine. The reaction with hydroxylamine was carried out
by gradual addition of acetic acid solution of 1,5-dicarbonyl compound to the
stirring refluxing suspension of hydroxylamine in glacial acetic acid. By usual
treatment was fractionned in vacuum to yield 37.5% of 2-methyl-5-nbutylpyridine,
boiling point 105°C.
The 2-methyl-5-n-butylpyridine was oxidated by selenium dioxide in pyridine
to 5-n-butyl-2-pyridine carboxylic (fusarinic) acid, melting point 100-101°C.
25.0 g of the 5-n-butyl-2-pyridine carboxylic (fusarinic) acid and 25 ml of
thionyl chloride were mixed; after all of the acid is dissolved, concentrate (in
vacuo) the mixture and take up the mixture in 500 ml of anhydrous benzene;
with cooling add the mixture to a solution of excess ammonia in 1 l of
benzene, concentrate (in vacuo) the resulting mixture; add water and
potassium carbonate and extract the amide with ether; dry and concentrate
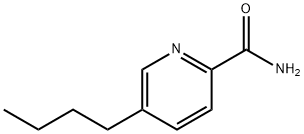
the ether extract and 5-n-butyl-2-pyridine carboxamide was obtained
(recrystallize from acetonitrile).