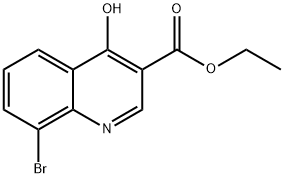
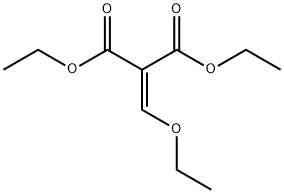
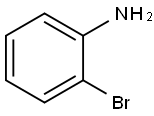
The reaction was carried out with 2-bromoaniline (2.5 g, 14.62 mmol) and diethyl ethoxymethylene malonate (3.16 g, 14.62 mmol) by heating at 100 °C for 3 hours. Subsequently, the volatiles in the reaction system were removed by a stream of nitrogen. The resulting melt was slowly added to boiling diphenyl ether (10 mL) and the mixture was heated to reflux for 2 hours. After completion of the reaction, the reaction mixture was allowed to cool to room temperature and petroleum ether was added to precipitate the product. The precipitated solid was collected by filtration and dried to give ethyl 4-hydroxy-8-bromoquinoline-3-carboxylate (3.5 g). The structure of the product was confirmed by 1H NMR (300 MHz, DMSO-d6): δ 11.65 (br s, 1H), 8.45 (s, 1H), 8.17 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.39-7.34 (t, J = 7.8 Hz, 1H), 4.26-4.19 (q, J = 7.2,14.1Hz, 2H), 1.30-1.26 (t, J = 6.9Hz, 3H).