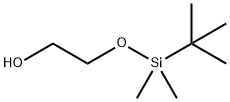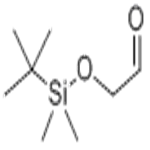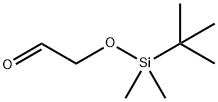
(TERT-BUTYLDIMETHYLSILYLOXY)ACETALDEHYDE
- Product Name(TERT-BUTYLDIMETHYLSILYLOXY)ACETALDEHYDE
- CAS102191-92-4
- MFC8H18O2Si
- MW174.31
- EINECS
- MOL File102191-92-4.mol
Chemical Properties
| Melting point | 165-167 ºC |
| Boiling point | 165-167 °C(lit.) |
| Density | 0.915 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | 140 °F |
| storage temp. | 2-8°C |
| form | Powder |
| color | White to light beige to grey |
| Specific Gravity | 0.915 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| InChI | InChI=1S/C8H18O2Si/c1-8(2,3)11(4,5)10-7-6-9/h6H,7H2,1-5H3 |
| InChIKey | MEBFFOKESLAUSJ-UHFFFAOYSA-N |
| SMILES | C(=O)CO[Si](C(C)(C)C)(C)C |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | UN 1989 3/PG 3 |
| WGK Germany | 3 |
| TSCA | No |
| HazardClass | 3 |
| HS Code | 29319090 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Eye Irrit. 2 Flam. Liq. 3 Skin Irrit. 2 STOT SE 3 |