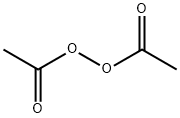
colourless liquid with a pungent odour.
Diacetyl peroxide as a commercial product is a colorless
liquid with a pungent odor. As a solid it is crystalline (sandlike)
with a sharp odor. The commercial product consists of a
25%solution of acetyl peroxide in dimethyl phthalate. It is an
organic peroxide and a carboxylic peracid derivative. It sinks
in water. Diacetyl peroxide is a colorless crystal. It must be
kept in solution because of its shock sensitivity and high
explosion risk.
Diacetyl peroxide diluted solutions with<25% strength are used as free-radicalsources to initiate polymerizations and inorganic syntheses.
Colorless liquid with a sharp odor. Consists of a solution of acetyl peroxide in dimethyl phthalate. Sinks in water.
Peroxides, such as ACETYL PEROXIDE, are good oxidizing agents. Organic compounds can ignite on contact with concentrated peroxides. Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides. There are few chemical classes that do not at least produce heat when mixed with peroxides. Many produce explosions or generate gases (toxic and nontoxic). Generally, dilute solutions of peroxides (<70%) are safe, but the presence of a catalyst (often a transition metal such as cobalt, iron, manganese, nickel, or vanadium) as an impurity may even then cause rapid decomposition, a buildup of heat, and even an explosion. Solutions of peroxides often become explosive when evaporated to dryness or near-dryness.
The concentrated compound is a severe eyehazard. An amount of 60 mg in a 1-minuterinse caused severe irritation in rabbit’s eyes.Skin contact of its solution may cause irritation.Toxicity of this compound should be oflow order. Diacetyl peroxide caused lung andblood tumors in rats. Its carcinogenic actionon humans is not reported.
Contact with liquid causes irritation of eyes and skin. If ingested, irritates mouth and stomach.
Behavior in Fire: May explode. Burns with accelerating intensity.
Severe skin and eye irritant. Questionable carcinogen with experimental tumorigenic data. Dangerous fire hazard by spontaneous chemical reaction. A powerful oxidizing agent; can cause ignition of organic materials on contact. Severe explosion hazard when shocked or exposed to heat. It may explode spontaneously in storage and should be used as soon as prepared. It will react with water or steam to produce heat; can react vigorously with reducing materials; emits toxic fumes on contact with acid or acid fumes. To fight fire, use CO2, dry chemical.
27' and not warmed over 30'. Do not add to hot materials. Do not add accelerator to this material. Store in origmal container with vented cap. Avoid bodily contact. This material is nearly always stored and handled as a 25% solution in an inert solvent. See also ACETYL PEROXIDE 25% solution (in dimethyl phthalate); and PEROXIDES, ORGANIC. Storage
Diacetyl peroxide is stored and shipped as a25% solution in dimethyl phthalate; the storagetemperature must not go down below-5°C (23°F) or above 32°C (89.6°F); it isstored in a cool and well-ventilated areaisolated from other chemicals and protectedfrom physical damage. Concentration, maximumto 25%, can be shipped in amber bottlesor polyethylene carboy containers with ventcaps not exceeding 10 and 45 lb.
It is ignited on the ground in a remoteoutdoor area using a long torch. Rinse theempty containers with 5–10% caustic sodaor caustic potash solution.